Abstract
Background:
Adipose tissue fibrosis is a relatively new notion and its relationship with visceral obesity and cardiometabolic alterations remains unclear, particularly in moderate obesity.
Objective:
Our objective was to examine if total and pericellular collagen accumulation are relevant for the pathophysiology of visceral obesity and related cardiometabolic risk.
Subjects and methods:
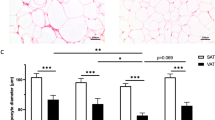
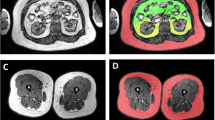
Surgical omental (OM) and subcutaneous (SC) fat samples were obtained in 56 women (age: 47.2±5.8 years; body mass index (BMI): 27.1±4.4 kg/m2). Body composition and fat distribution were measured by dual-energy X-ray absorptiometry and computed tomography, respectively. Total and pericellular collagen were measured using picrosirius red staining. CD68+ cells (total macrophages) and CD163+ cells (M2-macrophages) were identified using immunohistochemistry.
Results:
We found that only pericellular collagen percentage, especially in OM fat, was associated with higher BMI, body fat mass and adipose tissue areas as well as lower radiologic attenuation of visceral adipose tissue and altered cardiometabolic risk variables. Strong correlations between peri-adipocyte collagen percentage and total or M2-macrophage percentages were observed in both depots. Total collagen percentage in either compartment was not related to adiposity, fat distribution or cardiometabolic risk.
Conclusions:
As opposed to whole tissue-based assessments of adipose tissue fibrosis, collagen deposition around the adipocyte, especially in the OM fat compartment is related to total and regional adiposity as well as altered cardiometabolic risk profile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wellen KE, Hotamisligil GS . Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 2003; 112: 1785–1788.
Wellen KE, Hotamisligil GS . Inflammation, stress, and diabetes. J Clin Invest 2005; 115: 1111–1119.
Tchernof A, Despres JP . Pathophysiology of human visceral obesity: an update. Physiol Rev 2013; 93: 359–404.
Despres JP, Lemieux I . Abdominal obesity and metabolic syndrome. Nature 2006; 444: 881–887.
Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008; 28: 1039–1049.
Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010; 59: 105–109.
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O et al. Dynamics of fat cell turnover in humans. Nature 2008; 453: 783–787.
Drolet R, Richard C, Sniderman AD, Mailloux J, Fortier M, Huot C et al. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes 2008; 32: 283–291.
Sun K, Kusminski CM, Scherer PE . Adipose tissue remodeling and obesity. J Clin Invest 2011; 121: 2094–2101.
Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab 2013; 305: E1427–E1435.
Mariman EC, Wang P . Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci 2010; 67: 1277–1292.
Buechler C, Krautbauer S, Eisinger K . Adipose tissue fibrosis. World J Diabetes 2015; 6: 548–553.
Nakajima I, Muroya S, Tanabe R, Chikuni K . Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biol Cell 2002; 94: 197–203.
Sun K, Tordjman J, Clement K, Scherer PE . Fibrosis and adipose tissue dysfunction. Cell Metab 2013; 18: 470–477.
Chun TH, Peri-adipocyte ECM . remodeling in obesity and adipose tissue fibrosis. Adipocyte 2012; 1: 89–95.
Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 2009; 29: 1575–1591.
Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 2008; 9: R14.
Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010; 59: 2817–2825.
Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab 2009; 94: 5155–5162.
Tam CS, Tordjman J, Divoux A, Baur LA, Clement K . Adipose tissue remodeling in children: the link between collagen deposition and age-related adipocyte growth. J Clin Endocrinol Metab 2012; 97: 1320–1327.
Walker RW, Allayee H, Inserra A, Fruhwirth R, Alisi A, Devito R et al. Macrophages and fibrosis in adipose tissue are linked to liver damage and metabolic risk in obese children. Obesity 2014; 22: 1512–1519.
McCulloch LJ, Rawling TJ, Sjoholm K, Franck N, Dankel SN, Price EJ et al. COL6A3 is regulated by leptin in human adipose tissue and reduced in obesity. Endocrinology 2015; 156: 134–146.
Liu Y, Aron-Wisnewsky J, Marcelin G, Genser L, Le Naour G, Torcivia A et al. Accumulation and changes in composition of collagens in subcutaneous adipose tissue following bariatric surgery. J Clin Endocrinol Metab 2015; 101: 293–304.
Deschenes D, Couture P, Dupont P, Tchernof A . Subdivision of the subcutaneous adipose tissue compartment and lipid-lipoprotein levels in women. Obes Res 2003; 11: 469–476.
Michaud A, Drolet R, Noel S, Paris G, Tchernof A . Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism 2012; 61: 689–698.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Laforest S, Michaud A, Paris G, Pelletier M, Vidal H, Géloën A et alComparative analysis of three human adipocyte size measurement methods and their relevance for cardiometabolic risk Obesity 2016. (In press).
Abdennour M, Reggio S, Le Naour G, Liu Y, Poitou C, Aron-Wisnewsky J et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. J Clin Endocrinol Metab 2014; 99: 898–907.
Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005; 54: 2277–2286.
Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006; 55: 1554–1561.
Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. JLipid Res 2005; 46: 2347–2355.
Spencer M, Unal R, Zhu B, Rasouli N, McGehee Jr RE, Peterson CA et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 2011; 96: E1990–E1998.
Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab 2010; 299: E1016–E1027.
Dankel SN, Svard J, Mattha S, Claussnitzer M, Kloting N, Glunk V et al. COL6A3 expression in adipocytes associates with insulin resistance and depends on PPARgamma and adipocyte size. Obesity 2014; 22: 1807–1813.
Nakajima I, Muroya S, Tanabe R, Chikuni K . Positive effect of collagen V and VI on triglyceride accumulation during differentiation in cultures of bovine intramuscular adipocytes. Differentiation 2002; 70: 84–91.
Ibrahim MM . Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010; 11: 11–18.
Martinez-Santibanez G, Lumeng CN . Macrophages and the regulation of adipose tissue remodeling. Ann Rev Nutr 2014; 34: 57–76.
Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat Commun 2014; 5: 4982.
Gagnon A, Yarmo MN, Landry A, Sorisky A . Macrophages alter the differentiation-dependent decreases in fibronectin and collagen I/III protein levels in human preadipocytes. Lipids 2012; 47: 873–880.
Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D . Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol 2009; 23: 11–24.
Liu Y, Aron-Wisnewsky J, Marcelin G, Genser L, Le Naour G, Torcivia A et al. Accumulation and Changes in Composition of Collagens in Subcutaneous Adipose Tissue After Bariatric Surgery. J Clin Endocrinol Metab 2016; 101: 293–304.
Yeoh AJ, Pedley A, Rosenquist KJ, Hoffmann U, Fox CS . The association between subcutaneous fat density and the propensity to store fat viscerally. J Clin Endocrinol Metab 2015; 100: E1056–E1064.
Côté JA, Nazare JA, Nadeau M, Leboeuf M, Blackburn L, Despres JP et al. Computed tomography-measured adipose tissue area and attenuation predict fat cell size and cardiometabolic risk in women. Adipocyte 2015; 5: 35–42.
Acknowledgements
The study was supported by operating funds from the Canadian Institutes of Health Research (CIHR) to André Tchernof (MOP-102642). Andréanne Michaud is the recipient of a scholarship from Fonds de recherche du Québec-santé (FRQS) and a CIHR Institute of Gender and Health Gender, Sex and Health Research Skills Development Award, which was essential for this study. We also obtained funding from the Fondation pour la Recherche Médicale (FRM DEQ20120323701), the National Agency of Research (ANR Adipofib), the national program ‘Investissements d’avenir’. We would like to acknowledge the help of Marie-Frédérique Gauthier for the measurement of macrophage numbers. AT is the recipient of research grant support from Johnson & Johnson Medical Companies for studies unrelated to this publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Michaud, A., Tordjman, J., Pelletier, M. et al. Relevance of omental pericellular adipose tissue collagen in the pathophysiology of human abdominal obesity and related cardiometabolic risk. Int J Obes 40, 1823–1831 (2016). https://doi.org/10.1038/ijo.2016.173
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ijo.2016.173
This article is cited by
-
Adipocyte size, adipose tissue fibrosis, macrophage infiltration and disease risk are different in younger and older individuals with childhood versus adulthood onset obesity
International Journal of Obesity (2022)
-
Associations between markers of mammary adipose tissue dysfunction and breast cancer prognostic factors
International Journal of Obesity (2021)
-
Obesity phenotypes: depot-differences in adipose tissue and their clinical implications
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2018)
-
Histomorphometric analyses of human adipose tissues using intact, flash-frozen samples
Histochemistry and Cell Biology (2018)