Abstract
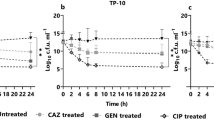
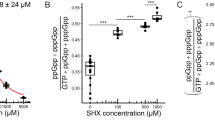
Biofilm formation of Pseudomonas aeruginosa on the surface of a reverse osmosis (RO) membrane was studied using a synthetic wastewater medium to simulate conditions relevant to reclamation of secondary wastewater effluent. P. aeruginosa biofilm physiology and spatial activity were analyzed following growth on the membrane using a short-life green fluorescent protein derivative expressed in a growth-dependent manner. As a consequence of the limiting carbon source prevailing in the suspended culture of the RO unit, a higher distribution of active cells was observed in the biofilm close to the membrane surface, likely due to the higher nutrient levels induced by concentration polarization effects. The faster growth of the RO-sessile cells compared to the planktonic cells in the RO unit was reflected by the transcriptome of the two cultures analyzed with DNA microarrays. In contrast to the findings recently reported in gene expression studies of P. aeruginosa biofilms, in the RO system, genes related to stress, adaptation, chemotaxis and resistance to antibacterial agents were induced in the planktonic cells. In agreement with the findings of previous P. aeruginosa biofilm studies, motility- and attachment-related genes were repressed in the RO P. aeruginosa biofilm. Supported by the microarray data, an increase in both motility and chemotaxis phenotypes was observed in the suspended cells. The increase in nutrient concentration in close proximity to the membrane is suggested to enhance biofouling by chemotaxis response of the suspended cells and their swimming toward the membrane surface.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Adler J . (1973). A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol 74: 77–91.
Ang WS, Lee S, Elimelech M . (2006). Chemical and physical aspects of cleaning of organic-fouled reverse osmosis membranes. J Membrane Sci 272: 198–210.
Aspedon A, Palmer K, Whiteley M . (2006). Microarray analysis of the osmotic stress response in Pseudomonas aeruginosa. J Bacteriol 188: 2721–2725.
Boles BR, Thoendel M, Singh PK . (2005). Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol 57: 1210–1223.
Bordes P, Repoila F, Kolb A, Gutierrez C . (2000). Involvement of differential efficiency of transcription by Eσs and Eσ70 RNA polymerase holoenzymes in growth phase regulation of the Escherichia coli osmE promoter. Mol Microbiol 35: 845–853.
Branda SS, Vik A, Friedman L, Kolter R . (2005). Biofilms: the matrix revisited. Trends Microbiol 13: 20–26.
Cha J, Cooksey D . (1991). Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc Natl Acad Sci USA 88: 8915–8919.
Chao G, Shen J, Tseng C, Park S, Gunsalus R . (1997). Aerobic regulation of isocitrate dehydrogenase gene (icd) expression in Escherichia coli by the arcA and fnr gene products. J Bacteriol 179: 4299–4304.
Costerton WJ, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM . (1995). Microbial biofilms. Annu Rev Microbiol 49: 711–745.
Danese PN, Pratt LA, Dove SL, Kolter R . (2000). The outer membrane protein, Antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol Microbiol 37: 424–432.
Davies DG, Chakrabarty AM, Geesey GG . (1993). Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl Environ Microbiol 59: 1181–1186.
Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP . (1998). The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280: 295–298.
Donlan RM . (2002). Biofilms: microbial life on surfaces. Emerging Infect Dis 8: 881–890.
Drenkard E . (2003). Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect 5: 1213–1219.
Friedman L, Kolter R . (2004). Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J Bacteriol 186: 4457–4465.
Fujita M, Tanaka K, Takahashi H, Amemura A . (1994). Transcription of the principal sigma-factor genes, rpoD and rpoS, in Pseudomonas aeruginosa is controlled according to the growth phase. Mol Microbiol 13: 1071–1077.
Gilbert P, Collier PJ, Brown MR . (1990). Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother 34: 1865–1868.
Gillis RJ, White KG, Choi K-H, Wagner VE, Schweizer HP, Iglewski BH . (2005). Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 49: 3858–3867.
Haagensen JAJ, Klausen M, Ernst RK, Miller SI, Folkesson A, Tolker-Nielsen T et al. (2006). Differentiation and distribution of colistin/SDS tolerant cells in Pseudomonas aeruginosa biofilms. J Bacteriol 189: 28–37.
Hagelueken G, Adams TM, Wiehlmann L, Widow U, Kolmar H, Tummler B et al. (2006). The crystal structure of SdsA1, an alkylsulfatase from Pseudomonas aeruginosa, defines a third class of sulfatases. Proc Natl Acad Sci USA 103: 7631–7636.
Hammer BK, Bassler BL . (2003). Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol 50: 101–104.
Hassett DJ, Elkins JG, Ma J-F, McDermott TR . (1999). Pseudomonas aeruginosa biofilm sensitivity to biocides: use of hydrogen peroxide as model antimicrobial agent for examining resistance mechanisms. Methods Enzymol 310: 599–608.
Herzberg M, Dosoretz CG, Kuhn J, Klein S, Green M . (2006). Visualization of active biomass distribution in a BGAC fluidized bed reactor using GFP tagged Pseudomonas putida F1. Water Res 40: 2704–2712.
Herzberg M, Elimelech M . (2007). Biofouling of reverse osmosis membranes: role of biofilm-enhanced osmotic pressure. J Membrane Sci 295: 11–20.
Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK et al. (2000). Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146: 2395–2407.
Jackson KD, Starkey M, Kremer S, Parsek MR, Wozniak DJ . (2004). Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J Bacteriol 186: 4466–4475.
Kirisits MJ, Parsek MR . (2007). Does Pseudomonas aeruginosa use intercellular signalling to build biofilm communities? Cell Microbiol 8: 1841–1849.
Lange R, Hengge-Aronis R . (1991). Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J Bacteriol 173: 4474–4481.
Liu Z, Stirling FR, Zhu J . (2007). Temporal quorum-sensing induction regulates Vibrio cholerae biofilm architecture. Infect Immun 75: 122–126.
Malone AS, Chung Y-K, Yousef AE . (2006). Genes of Escherichia coli O157:H7 that are involved in high-pressure resistance. Appl Environ Microbiol 72: 2661–2671.
Mikkelsen H, Duck Z, Lilley KS, Welch M . (2007). Interrelationships between colonies, biofilms, and planktonic cells of Pseudomonas aeruginosa. J Bacteriol 189: 2411–2416.
Morgan R, Kohn S, Hwang S-H, Hassett DJ, Sauer K . (2006). BdlA, a chemotaxis regulator essential for biofilm dispersion in Pseudomonas aeruginosa. J Bacteriol 188: 7335–7343.
O'Toole GA, Kaplan HB, Kolter R . (2000). Biofilm formation as microbial development. Annu Rev Microbiol 54: 49–79.
O'Toole GA, Kolter R . (1998). Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30: 295–304.
Parsek MR, Greenberg EP . (2005). Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13: 27–33.
Peng X, Xu C, Ren H, Lin X, Wu L, Wang S . (2005). Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Pseudomonas aeruginosa responding to ampicilin, kanamycin, and tetracycline resistance. J Proteome Res 4: 2257–2265.
Pettygrove GS, Asano T . (1984). Irrigation with reclaimed municipal wastewater: a guidance manual. California State Water Resources Control Board: Sacramento, CA (http://cee.engr.ucdavis.edu/Faculty/asano/IrrigationReuse1.pdf).
Ramsey MM, Whiteley M . (2004). Pseudomonas aeruginosa attachment and biofilm development in dynamic environments. Mol Microbiol 53: 1075–1087.
Rensing C, Pribyl T, Nies D . (1997). New functions for the three subunits of the CzcCBA cation-proton antiporter. J Bacteriol 179: 6871–6879.
Ridgway HF, Flemming H-C . (1996). Membrane Biofouling in Water Treatment Membrane Processes. In: Water Treatment Membrane Processes. McGraw-Hill, New York.
Rivera M, Hancock RE, Sawyer JG, Haug A, McGroarty EJ . (1988). Enhanced binding of polycationic antibiotics to lipopolysaccharide from an aminoglycoside-supersusceptible, tolA mutant strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother 32: 649–655.
Romeo T . (2006). When the party is over: a signal for dispersal of Pseudomonas aeruginosa biofilms. J Bacteriol 188: 7325–7327.
Sauer K, Camper AK . (2001). Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J Bacteriol 183: 6579–6589.
Sauer K, Camper AK, Ehrlich GD, Costerton JW, Davies DG . (2002). Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J Bacteriol 184: 1140–1154.
Sauer K, Cullen MC, Rickard AH, Zeef LAH, Davies DG, Gilbert P . (2004). Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J Bacteriol 186: 7312–7326.
Schembri MA, Christiansen G, Klemm P . (2001). FimH-mediated autoaggregation of Escherichia coli. Mol Microbiol 41: 1419–1430.
Schembri MA, Kjærgaard K, Klemm P . (2003). Global gene expression in Escherichia coli biofilms. Mol Microbiol 48: 253–267.
Schuster M, Hawkins AC, Harwood CS, Greenberg EP . (2004). The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol Microbiol 51: 973–985.
Shah D, Zhang Z, Khodursky A, Kaldalu N, Kurg K, Lewis K . (2006). Persisters: a distinct physiological state of E. coli. BMC Microbiol 6: 53.
Southey-Pillig CJ, Davies DG, Sauer K . (2005). Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. J Bacteriol 187: 8114–8126.
Sperandio V, Torres AG, Kaper JB . (2002). Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in Escherichia coli. Mol Microbiol 43: 809–821.
Sternberg C, Christensen BB, Johansen T, Toftgaard Nielsen A, Andersen JB, Givskov M et al. (1999). Distribution of bacterial growth activity in flow-chamber biofilms. Appl Environ Microbiol 65: 4108–4117.
Stewart PS . (2002). Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol 292: 107–113.
Strathmann M, Wingender J, Flemming H-C . (2002). Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J Microbiol Methods 50: 237–248.
Tamber S, Ochs MM, Hancock REW . (2006). Role of the novel OprD family of porins in nutrient uptake in Pseudomonas aeruginosa. J Bacteriol 188: 45–54.
Vieira HLA, Freire P, Arraiano CM . (2004). Effect of Escherichia coli morphogene bolA on biofilms. Appl Environ Microbiol 70: 5682–5684.
Vrijenhoek EM, Hong S, Elimelech M . (2001). Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J Membrane Sci 188: 115–128.
Waite R, Paccanaro A, Papakonstantinopoulou A, Hurst J, Saqi M, Littler E et al. (2006). Clustering of Pseudomonas aeruginosa transcriptomes from planktonic cultures, developing and mature biofilms reveals distinct expression profiles. BMC Genomics 7: 162.
Waite RD, Papakonstantinopoulou A, Littler E, Curtis MA . (2005). Transcriptome analysis of Pseudomonas aeruginosa growth: comparison of gene expression in planktonic cultures and developing and mature biofilms. J Bacteriol 187: 6571–6576.
Wang X, Preston III JF, Romeo T . (2004). The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 186: 2724–2734.
Wei X, Bauer WD . (1998). Starvation-induced changes in motility, chemotaxis, and flagellation of Rhizobium meliloti. Appl Environ Microbiol 64: 1708–1714.
Werner E, Roe F, Bugnicourt A, Franklin MJ, Heydorn A, Molin S et al. (2004). Stratified growth in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 70: 6188–6196.
Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S et al. (2001). Gene expression in Pseudomonas aeruginosa biofilms. Nature 413: 860–864.
Winsor GL, Lo R, Sui SJH, Ung KSE, Huang S, Cheng D et al. (2005). Pseudomonas aeruginosa Genome Database and PseudoCAP: facilitating community-based, continually updated, genome annotation. Nucleic Acids Res 33: D338–D343.
Xu KD, Franklin MJ, Park C-H, McFeters GA, Stewart PS . (2001). Gene expression and protein levels of the stationary phase sigma factor, RpoS, in continuously-fed Pseudomonas aeruginosa biofilms. FEMS Microbiol Lett 199: 67–71.
Acknowledgements
Financial support for this study was provided by the WaterCAMPWS, a Science and Technology Center of Advanced Materials for the Purification of Water with Systems under the National Science Foundation agreement number CTS-0120978, and by a postdoctoral fellowship (to MH) from the United States–Israel Binational Agricultural Research and Development (BARD) fund. We are also grateful to Professor S Molin from the Technical University of Denmark for providing us with Pseudomonas aeruginosa PAO1 AH298.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary information
Rights and permissions
About this article
Cite this article
Herzberg, M., Elimelech, M. Physiology and genetic traits of reverse osmosis membrane biofilms: a case study with Pseudomonas aeruginosa. ISME J 2, 180–194 (2008). https://doi.org/10.1038/ismej.2007.108
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2007.108
Keywords
This article is cited by
-
Isolation and characterization of Sphingomonadaceae from fouled membranes
npj Biofilms and Microbiomes (2019)
-
Impacts of biofouling on the removal of pharmaceutically active compounds by a nanofiltration membrane
Environmental Science and Pollution Research (2019)
-
Nanopatterning of steel by one-step anodization for anti-adhesion of bacteria
Scientific Reports (2017)
-
Cellulose effects on morphology and elasticity of Vibrio fischeri biofilms
npj Biofilms and Microbiomes (2016)
-
Biofouling ecology as a means to better understand membrane biofouling
Applied Microbiology and Biotechnology (2014)