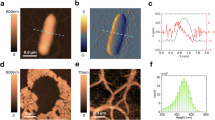
Abstract
In microbial oceanography, cell size, volume and carbon (C) content of pelagic bacteria and archaea (‘bacteria’) are critical parameters in addressing the in situ physiology and functions of bacteria, and their role in the food web and C cycle. However, because of the diminutive size of most pelagic bacteria and errors caused by sample fixation and processing, an accurate measurement of the size and volume has been challenging. We used atomic force microscopy (AFM) to obtain high-resolution images of pelagic bacteria and Synechococcus. We measured the length, width and height of live and formalin-fixed pelagic bacteria, and computed individual cell volumes. AFM-based measurements were compared with those by epifluorescence microscopy (EFM) using 4′,6-diamidino-2-phenylindole (DAPI). The ability to measure cell height by AFM provides methodological advantage and ecophysiological insight. For the samples examined, EFM (DAPI)-based average cell volume was in good agreement (1.1-fold) with live sample AFM. However, the agreement may be a fortuitous balance between cell shrinkage due to fixation/drying (threefold) and Z-overestimation (as EFM does not account for cell flattening caused by sample processing and assumes that height=width). The two methods showed major differences in cell volume and cell C frequency distributions. This study refines the methodology for quantifying bacteria-mediated C fluxes and the role of bacteria in marine ecosystems, and suggests the potential of AFM for individual cell physiological interrogations in natural marine assemblages.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Ahlgren NA, Rocap G . (2006). Culture isolation and culture-independent clone libraries reveal new marine Synechococcus ecotypes with distinctive light and N physiologies. Appl Environ Microbiol 72: 7193–7204.
Amro NA, Kotra LP, Wadu-Mesthrige K, Bulychev A, Mobashery S, Liu GY . (2000). High-resolution atomic force microscopy studies of the escherichia coli outer membrane: structural basis for permeability. Langmuir 16: 2789–2796.
Azam F, Malfatti F . (2007). Microbial structuring of marine ecosystems. Nat Rev Microbiol 5: 782–791.
Azam F, Smith DC, Steward GF, Hagström Å . (1994). Bacteria-organic matter coupling and its significance for oceanic carbon cycling. Microb Ecol 28: 167–179.
Binning G, Quate CF . (1986). Atomic force microscopy. Phys Rev Lett 56: 930–933.
Boelter M, Bloem J, Meiners K, Moller R . (2002). Enumeration and biovolume determination of microbial cells - a methodological review and recommendations for applications in ecological research. Biol Fertil Soils 36: 249–259.
Bouvier T, del Giorgio PA, Gasol JM . (2007). A comparative study of the cytometric characteristics of high and low nucleic-acid bacterioplankton cells from different aquatic ecosystems. Environ Microbiol 9: 2050–2066.
Braga PC, Ricci D . (1998). Atomic force microscopy: application to investigation of Escherichia coli morphology before and after exposure to cefodizime. Antimicrob Agents Chemother 42: 18–22.
Bratbak G . (1985). Bacteria biovolume and biomass estimations. Appl Environ Microbiol 49: 1488–1493.
Cabeen MT, Jacobs-Wagner C . (2005). Bacterial cell shape. Nat Rev Microbiol 3: 601–610.
Camesano AT, Natan MJ, Logan BE . (2000). Observation of changes in bacterial cell morphology using tapping mode atomic force microscopy. Langmuir 16: 4563–4572.
Christaki U, Jacquet S, Dolan JR, Vaulot D, Rassoulzadegan F . (1999). Differential grazing and growth on Prochlorococcus and Synechococcus by two contrasting ciliates. Limnol Oceanogr 44: 52–61.
Christaki U, Vazquez-DomÌnguez E, Courties C, Lebaron P . (2005). Grazing impact of different heterotrophic nanoflagellates on eukaryotic Ostreococcus tauri and prokaryotic picoautotrophs Prochlorococcus and Synechococcus. Environ Microbiol 7: 1200–1210.
Corno G, Jurgens K . (2006). Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl Environ Microbiol 72: 78–86.
Cosa G, Focsaneanu KS, McLean JRN, McNamee JP, Scaiano JC . (2001). Photophysical properties of fluorescent dna-dyes bound to single- and double-stranded dna in aqueous buffered solution. Photochem Photobiol 73: 585–599.
Costerton JW, Irvin RT, Cheng KJ . (1981). The bacterial glycocalyx in nature and disease. Ann Rev Microbiol 35: 299–324.
del Giorgio PA, Gasol JM . (2008). Physiological structure and single-cell activity in marine bacterioplankton. In: Kirchman DL (ed). Microbial Ecology of the Oceans. John Wiley & Sons Inc.: Hoboken, NJ, pp 243–298.
del Giorgio PA, Williams PJlB (eds). (2005). The global significance of respiration in aquatic ecosystems: From single cells to the biosphere. In: Respiration in Aquatic Ecosystems. Oxford University Press: New York, pp 2667–2673.
Dubrovin EV, Voloshin AG, Kraevsky SV, Ignatyuk TE, Abramchuk SS, Yaminsky IV et al. (2008). Atomic force microscopy investigation of phage infection of bacteria. Langmuir 24: 13068–13074.
Dufrene YF . (2002). Atomic force microscopy, a powerful tool in microbiology. J Bacteriol 184: 5205–5213.
Dufrene YF . (2008). Atomic force microscopy and chemical force microscopy of microbial cells. Nat Protoc 3: 1132–1138.
Falcioni T, Papa S, Gasol JM . (2008). Evaluating the flow-cytometry nucleic acid double staining protocol in realistic situations of planktonic bacterial death. Appl Environ Microbiol 74: 1767–1779.
Fuhrman JA . (1981). Influence of method on the apparent size distribution of bacterioplankton cell: epifluorescence microscopy compared to scanning electron microscopy. Mar Ecol Prog Ser 5: 103–106.
Fuhrman JA . (1999). Marine viruses and their biogeochemical and ecological effects. Nature 399: 541–548.
Fuhrman JA, Azam F . (1980). Bacterioplankton secondary production estimates for coastal waters of British Columbia, Antarctica and California. Appl Environ Microbiol 39: 1085–1095.
Fuhrman JA, Azam F . (1982). Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar Biol 66: 109–120.
Garrison DL, Gowing MM, Hughes MP, Campbell L, Caron DA, Dennett MR et al. (2000). Microbial food web structure in the Arabian Sea: a US JGOFS study. Deep Sea Res Part 2 Top Stud Oceanogr 47: 1387–1422.
Gundersen K, Heldal M, Norland S, Purdie DA, Knap AH . (2002). Elemental C, N, and P cell content of individual bacteria collected at the Bermuda Atlantic Time-series Study (BATS) site. Limnol Oceanogr 47: 1525–1530.
Gustavson KH . (1956). The Chemistry of Tanning Processes. Academic Press: New York.
Heissenberger A, Leppard G, Herndl G . (1996). Ultrastructure of marine snow. II. Microbiological considerations. Mar Ecol Prog Ser 135: 299–308.
Higgins MJ, Crawford SA, Mulvaney P, Wetherbee R . (2002). Characterization of the adhesive mucilages secreted by live diatom cells using atomic force microscopy. Protist 153: 25–38.
Hobbie JE, Daley RJ, Jasper S . (1977). Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33: 1225–1228.
Johnson PW, Sieburth JM . (1982). In-situ morphology and occurrence of eukaryotic phototrophs of bacterial size in the picoplankton of estuarine and oceanic waters. J Phycol 18: 318–327.
Kubitschek HE . (1969). Counting and sizing micro-organisms with the Coulter Counter. Methods Microbiol 1: 593–610.
Lee S, Fuhrman JA . (1987). Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl Environ Microbiol 53: 1298–1303.
Malfatti F, Azam F . (2009). Atomic force microscopy reveals microscale networks and possible symbioses among pelagic marine bacteria. Aquat Microb Ecol (in press).
Meincken M, Holroyd DL, Rautenbach M . (2005). Atomic force microscopy study on the effect of antimicrobial peptides on the cell envelope of Escherichia coli. Antimicrob Agents Chemother 49: 4085–4092.
Mitchell J . (1991). The influence of cell size on marine bacterial motility and energetics. Microb Ecol 22: 227–238.
Nie CL, Yan Wei Y, Chen X, Liu YY, Dui W, Liu Y et al. (2007). Formaldehyde at low concentration induces protein tau into globular amyloid-like aggregates in vitro and in vivo. Plos ONE 2: e629.
Nishino T, Ikemoto E, Kazuhiro K . (2004). Application of atomic force microscopy to observation of marine bacteria. J Oceanogr 60: 219–225.
Palenik B, Ren Q, Dupont CL, Myers GS, Heidelberg JF, Badger JH et al. (2006). Genome sequence of Synechococcus CC9311: Insights into adaptation to a coastal environment. Proc Natl Acad Sci 103: 13555–13559.
Pomeroy LR, Williams PJL, Azam F, Hobbie JE . (2007). The microbial loop. Oceanography 20: 28–33.
Posch T, Loferer-Kroessbacher M, Gao G, Alfreider A, Pernthaler J, Psenner R . (2001). Precision of bacterioplankton biomass determination: a comparison of two fluorescent dyes, and of allometric and linear volume-to-carbon conversion factor. Aquat Microb Ecol 25: 55–63.
Posch T, Pernthaler J, Alfreider A, Psenner R . (1997). Cell-specific respiratory activity of aquatic bacteria studied with the tetrazolium reduction method, cyto-clear slides, and image analysis. Appl Environ Microbiol 63: 867–873.
Proctor LM, Fuhrman JA . (1990). Viral mortality of marine bacteria and cyanobacteria. Nature 343: 60–62.
Seo Y, Eiko I, Akihiro Y, Kazuhiro K . (2007). Particle capture by marine bacteria. Aquat Microb Ecol 49: 243–253.
Sieburth JM . (1975). Microbial Seascape: A Pictorial Essay On Marine Microorganisms And Their Environments. University Park Press: Baltimore.
Sieracki ME, Johnson PW, Seiburth JM . (1985). Detection, enumeration, and sizing of planktonic bacteria by image-analyzed epifluorescence microscopy. Appl Environ Microbiol 49: 799–810.
Simek K, Chrzanowski TH . (1992). Direct and indirect evidence of size-selective grazing on pelagic bacteria by freshwater nanoflagellates. Appl Environ Microbiol 58: 3715–3720.
Simon M, Azam F . (1989). Protein content and protein synthesis rates of planktonic bacteria. Mar Ecol Prog Ser 51: 201–213.
Steward GF, Wikner J, Smith DC, Cochlan WP, Azam F . (1992). Estimation of virus production in the sea: I. Method development. Mar Microbial Food Webs 6: 57–78.
Straza TRA, Cottrell MT, Ducklow HW, Kirchman DL . (2009). Geographic and phylogenetic variation in bacterial biovolume using protein and nucleic acid staining. Appl Environ Microbiol 75: 4028–4034.
Suzuki MT, Sherr EB, Sherr BF . (1993). DAPI direct counting underestimates bacterial abundances and average cell size compared to AO direct counting. Limnol Oceanogr 38: 1566–1570.
Wommack KE, Hill RT, Kesel M, Russek-Cohen E, Colwell R . (1992). Distribution of viruses in the Chesapeacke Bay. Appl Environ Microbiol 58: 2965–2970.
Wong C, West P, Olson K, Mecartney M, Starostina N . (2007). Tip dilation and AFM capabilities in the characterization of nanoparticles. JOM J Miner Met Mater Soc 59: 12–16.
Zweifel UL, Hagström A . (1995). Total counts of marine bacteria include a large fraction of non-nucleoid-containing bacteria (Ghosts). Appl Environ Microbiol 61: 2180–2185.
Acknowledgements
We thank Maura Manganelli, Jessica Ward and Anne-Clarie Baudoux for kindly providing samples from BWZ II cruise 2006 in Antarctica, CCE LTER Process Cruise 2007 and from the SWAT3 phage experiment. We thank Krystal Rypien for her help on the statistical test. We thank Katherine Barbeau and Melissa Garren for their comments. We thank Asylum Research folks for AFM support. Chlorophyll and temperature data were retrieved from the SCCOOS data archive (www.sccoos.org) supported by NOAA, from the CCE LTER data archive (http://oceaninformatics.ucsd.edu/datazoo/data) supported by the Division of Ocean Sciences, NSF Grant OCE-0417616 and from the BWZ II website supported by NSF Grant ANT- 0444134 to FA. This work was supported by grants from the Gordon and Betty Moore Foundation Marine Microbiology Initiative and from NSF to FA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary information
Rights and permissions
About this article
Cite this article
Malfatti, F., Samo, T. & Azam, F. High-resolution imaging of pelagic bacteria by Atomic Force Microscopy and implications for carbon cycling. ISME J 4, 427–439 (2010). https://doi.org/10.1038/ismej.2009.116
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2009.116
Keywords
This article is cited by
-
The relationship between two Synechococcus strains and heterotrophic bacterial communities and its associated carbon flow
Journal of Applied Phycology (2021)
-
Competitive strategies differentiate closely related species of marine actinobacteria
The ISME Journal (2016)
-
Cross-depth analysis of marine bacterial networks suggests downward propagation of temporal changes
The ISME Journal (2015)
-
Micro-friction of diazoresin/polyacrylic acid self-assembly films in water with atomic force microscopy
Wuhan University Journal of Natural Sciences (2014)
-
Response of Prochlorococcus ecotypes to co-culture with diverse marine bacteria
The ISME Journal (2011)