Abstract
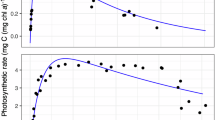
Here we present, to the best of our knowledge, the first balanced light energy budget for a benthic microbial mat ecosystem, and show how the budget and the spatial distribution of the local photosynthetic efficiencies within the euphotic zone depend on the absorbed irradiance (Jabs). Our approach uses microscale measurements of the rates of heat dissipation, gross photosynthesis and light absorption in the system, and a model describing light propagation and conversion in a scattering–absorbing medium. The energy budget was dominated by heat dissipation on the expense of photosynthesis: in light-limiting conditions, 95.5% of the absorbed light energy dissipated as heat and 4.5% was channeled into photosynthesis. This energy disproportionation changed in favor of heat dissipation at increasing irradiance, with >99% of the absorbed light energy being dissipated as heat and <1% used by photosynthesis at Jabs>700 μmol photon m−2 s−1 (>150 J m−2 s−1). Maximum photosynthetic efficiencies varied with depth in the euphotic zone between 0.014−0.047 O2 per photon. Owing to steep light gradients, photosynthetic efficiencies varied differently with increasing irradiances at different depths in the euphotic zone; for example, at Jabs>700 μmol photon m−2 s−1, they reached around 10% of the maximum values at depths 0−0.3 mm and progressively increased toward 100% below 0.3 mm. This study provides the base for addressing, in much more detail, the photobiology of densely populated photosynthetic systems with intense absorption and scattering. Furthermore, our analysis has promising applications in other areas of photosynthesis research, such as plant biology and biotechnology.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aarti D, Tanaka R, Ito H, Tanaka A . (2007). High light inhibits chlorophyll biosynthesis at the level of 5-aminolevulinate synthesis during de-etiolation in cucumber (Cucumis sativus) cotyledons. Photochem Photobiol 83: 171–176.
Cahoon LB . (1999). The role of benthic microalgae in neritic ecosystems. Oceanogr Mar Biol 37: 47–86.
Dismukes GC, Carrieri D, Bennette N, Ananyev GM, Posewitz MC . (2008). Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr Opin Biotechnol 19: 235–240.
Dubinsky Z, Falkowski PG, Wyman K . (1986). Light harvesting and utilization by phytoplankton. Plant Cell Physiol 27: 1335–1349.
Falkowski PG, Raven JA . (1997). Aquatic photosynthesis. Blackwell Science. Capital City Press: Washington, DC.
Flameling IA, Kromkamp J . (1998). Light dependence of quantum yields for PSII charge separation and oxygen evolution in eucaryotic algae. Limnol Oceanogr 43: 284–297.
Guerrero R, Piqueras M, Berlanga M . (2002). Microbial mats and the search for minimal ecosystems. Int Microbiol 5: 177–188.
Hihara Y, Kamei A, Kanehisa M, Kaplan A, Ikeuchi M . (2001). DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13: 793–806.
Huner NPA, Oquist G, Sarhan F . (1998). Energy balance and acclimation to light and cold. Trends Plant Sci 3: 224–230.
Jimenez IM, Kühl M, Larkum AWD, Ralph PJ . (2008). Heat budget and thermal microenvironment of shallow-water corals: do massive corals get warmer than branching corals? Limnol Oceanogr 53: 1548–1561.
Jørgensen BB, Cohen Y, des Marais DJ . (1987). Photosynthetic action spectra and adaptation to spectral light-distribution in a benthic cyanobacterial mat. Appl Environmental Microbiol 53: 879–886.
Jørgensen BB, des Marais DJ . (1988). Optical-properties of benthic photosynthetic communities—fiber-optic studies of cyanobacterial mats. Limnol Oceanogr 33: 99–113.
Kühl M . (2005). Optical microsensors for analysis of microbial communities. Methods Enzymol 397: 166–199.
Kühl M, Fenchel T . (2000). Bio-optical characteristics and the vertical distribution of photosynthetic pigments and photosynthesis in an artificial cyanobacterial mat. Microb Ecol 40: 94–103.
Kühl M, Glud RN, Ploug H, Ramsing NB . (1996). Microenvironmental control of photosynthesis and photosynthesis-coupled respiration in an epilithic cyanobacterial biofilm. J Phycol 32: 799–812.
Kühl M, Jørgensen BB . (1992). Spectral light measurements in microbenthic phototrophic communities with a fiber-optic microprobe coupled to a sensitive diode array detector. Limnol Oceanogr 37: 1813–1823.
Kühl M, Jørgensen BB . (1994). The light-field of microbenthic communities—radiance distribution and microscale optics of sandy coastal sediments. Limnol Oceanogr 39: 1368–1398.
Kühl M, Lassen C, Jørgensen BB . (1994). Light penetration and light intensity in sandy marine sediments measured with irradiance and scalar irradiance fiber-optic microprobes. Mar Ecol Prog Ser 105: 139–148.
Kühl M, Polerecky L . (2008). Functional and structural imaging of phototrophic microbial communities and symbioses. Aquat Microb Ecol 53: 99–118.
Lassen C, Ploug H, Jørgensen BB . (1992a). A fiberoptic scalar irradiance microsensor—application for spectral light measurements in sediments. FEMS Microbiol Ecol 86: 247–254.
Lassen C, Ploug H, Jørgensen BB . (1992b). Microalgal photosynthesis and spectral scalar irradiance in coastal marine-sediments of Limfjorden, Denmark. Limnol Oceanogr 37: 760–772.
Latifi A, Ruiz M, Zhang CC . (2009). Oxidative stress in cyanobacteria. FEMS Microb Rev 33: 258–278.
MacIntyre HL, Kana TM, Anning T, Geider RJ . (2002). Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J Phycol 38: 17–38.
Makarieva AM, Gorshkov VG, Li BL . (2008). Energy budget of the biosphere and civilization: rethinking environmental security of global renewable and non-renewable resources. Ecol Complex 5: 281–288.
Mussgnug JH, Thomas-Hall S, Rupprecht J, Foo A, Klassen V, McDowall A et al. (2007). Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion. Plant Biotechnol J 5: 802–814.
Nishiyama Y, Allakhverdiev SI, Yamamoto H, Hayashi H, Murata N . (2004). Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp PCC 6803. Biochemistry 43: 11321–11330.
Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N . (2001). Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20: 5587–5594.
Osmond CB . (1994). What is photoinhibition? Some insights from comparisons of shade and sun plants. In: NR Baker and JR Bowyer (eds). Environmental Plant Biology Series; Photoinhibition of photosynthesis: From molecular mechanisms to the field. Bios Scientific Publisher: Oxford. pp 1–24.
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA et al. (2006). The path forward for biofuels and biomaterials. Science 311: 484–489.
Revsbech NP . (1989). An oxygen microsensor with a guard cathode. Limnol Oceanogr 34: 474–478.
Revsbech NP, Jørgensen BB . (1983). Photosynthesis of benthic microflora measured with high spatial-resolution by the oxygen microprofile method—capabilities and limitations of the method. Limnol Oceanogr 28: 749–756.
Revsbech NP, Jørgensen BB . (1986). Microelectrodes—Their use in microbial ecology. Adv Microb Ecol 9: 293–352.
Revsbech NP, Jørgensen BB, Blackburn TH, Cohen Y . (1983). Microelectrode studies of the photosynthesis and O2, H2S, and pH profiles of a microbial mat. Limnol Oceanogr 28: 1062–1074.
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ . (2008). A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19: 430–436.
Schneider TR . (1973). Efficiency of photosynthesis as a solar energy converter. Energy Conversion 13: 77–84.
Schreiber U, Kühl M, Klimant I, Reising H . (1996). Measurement of chlorophyll fluorescence within leaves using a modified PAM Fluorometer with a fiber-optic microprobe. Photosynth Res 47: 103–109.
Singsaas EL, Ort DR, DeLucia EH . (2001). Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia 128: 15–23.
Stal LJ . (2000). Cyanobacterial mats and stromatolites. In: AB Whitton and M Potts (eds). The Ecology of Cyanobacteria. Kluwer Academic Publishers: Dordrecht. pp 61–120.
Thauer RK, Jungermann K, Decker K . (1977). Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41: 100–180.
vanGemerden H . (1993). Microbial mats: a joint venture. Mar Geol 113: 3–25.
Webb WL, Newton M, Starr D . (1974). Carbon-dioxide exchange of Alnus-Rubra—mathematical model. Oecologia 17: 281–291.
Yang L, Kruse B, Miklavcic SJ . (2004). Revised Kubelka–Munk theory. II. Unified framework for homogeneous and inhomogeneous optical media. J Opt Soc Am A Opt Image Sci Vis 21: 1942–1952.
Zhu XG, Long SP, Ort DR . (2008). What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 19: 153–159.
Acknowledgements
We thank the technicians of the microsensor group for microsensor construction and Paul Faerber, Harald Osmers, Georg Herz and Volker Meyer for their technical support. We thank Dr Henk Jonkers and Prof Friedrich Widdel for fruitful discussions, and Prof Waleed Hamza for his guidance and assistance during sampling. Two anonymous reviewers are specially thanked for their useful comments and suggestions to improve the paper. This study was financially supported by the Max Planck Society (to DB, BBJ and LP), the Yusef Jameel foundation (to MA-N) and the Danish Natural Science Research Council (to MK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary information
Rights and permissions
About this article
Cite this article
Al-Najjar, M., de Beer, D., Jørgensen, B. et al. Conversion and conservation of light energy in a photosynthetic microbial mat ecosystem. ISME J 4, 440–449 (2010). https://doi.org/10.1038/ismej.2009.121
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2009.121
Keywords
This article is cited by
-
Radiative Energy Budgets in a Microbial Mat Under Different Irradiance and Tidal Conditions
Microbial Ecology (2019)
-
Structure and function of natural sulphide-oxidizing microbial mats under dynamic input of light and chemical energy
The ISME Journal (2016)
-
Heat generation and light scattering of green fluorescent protein-like pigments in coral tissue
Scientific Reports (2016)
-
A phototrophy-driven microbial food web in a rice soil
Journal of Soils and Sediments (2011)