Abstract
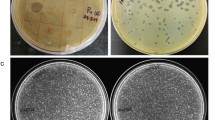
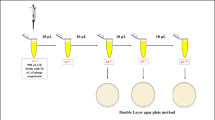
Viruses were earlier found to be 10-fold more abundant than prokaryotes in deep granitic groundwater at the Äspö Hard Rock Laboratory (HRL). Using a most probable number (MPN) method, 8–30 000 cells of sulphate-reducing bacteria per ml were found in groundwater from seven boreholes at the Äspö HRL. The content of lytic phages infecting the indigenous bacterium Desulfovibrio aespoeensis in Äspö groundwater was analysed using the MPN technique for phages. In four of 10 boreholes, 0.2−80 phages per ml were found at depths of 342–450 m. Isolates of lytic phages were made from five cultures. Using transmission electron microscopy, these were characterized and found to be in the Podoviridae morphology group. The isolated phages were further analysed regarding host range and were found not to infect five other species of Desulfovibrio or 10 Desulfovibrio isolates with up to 99.9% 16S rRNA gene sequence identity to D. aespoeensis. To further analyse phage–host interactions, using a direct count method, growth of the phages and their host was followed in batch cultures, and the viral burst size was calculated to be ∼170 phages per lytic event, after a latent period of ∼70 h. When surviving cells from infected D. aespoeensis batch cultures were inoculated into new cultures and reinfected, immunity to the phages was found. The parasite–prey system found implies that viruses are important for microbial ecosystem diversity and activity, and for microbial numbers in deep subsurface groundwater.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anonymous (1995) Agency USEP (ed) Virus monitoring protocol for the information collection requirements rule. Government Printing Office: Cincinnati, OH.
Bale SJ, Goodman K, Rochelle PA, Marchesi JR, Fry JC, Weightman AJ et al. (1997). Desulfovibrio profundus sp. nov., a novel barophilic sulfate-reducing bacterium from deep sediment layers in the Japan Sea. Int J Syst Bacteriol 47: 515–521.
Chen F, Lu JR, Binder BJ, Liu YC, Hodson RE . (2001). Application of digital image analysis and flow cytometry to enumerate marine viruses stained with SYBR gold. Appl Environ Microbiol 67: 539–545.
Chibani-Chennoufi S, Bruttin A, Dillmann ML, Brussow H . (2004). Phage–host interaction: an ecological perspective. J Bacteriol 186: 3677–3686.
Danovaro R, Dell’Anno A, Corinaldesi C, Magagnini M, Noble R, Tamburini C et al. (2008). Major viral impact on the functioning of benthic deep-sea ecosystems. Nature 454: 1084–1087.
Debruijn FJ . (1992). Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain-reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol 58: 2180–2187.
Eydal HSC, Pedersen K . (2007). Use of an ATP assay to determine viable microbial biomass in Fennoscandian Shield groundwater from depths of 3–1000 m. J Microbiol Methods 70: 363–373.
Fru EC, Athar R . (2008). In situ bacterial colonization of compacted bentonite under deep geological high-level radioactive waste repository conditions. Appl Microbiol Biot 79: 499–510.
Fuhrman JA . (1999). Marine viruses and their biogeochemical and ecological effects. Nature 399: 541–548.
Hallbeck L, Pedersen K . (2008). Characterization of microbial processes in deep aquifers of the Fennoscandian Shield. Appl Geochem 23: 1796–1819.
Handley J, Adams V, Akagi JM . (1973). Morphology of bacteriophage-like particles from Desulfovibrio vulgaris. J Bacteriol 115: 1205–1207.
Jensen EC, Schrader HS, Rieland B, Thompson TL, Lee KW, Nickerson KW et al. (1998). Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl Environ Microbiol 64: 575–580.
Jiang SC, Paul JH . (1998). Significance of lysogeny in the marine environment: studies with isolates and a model of lysogenic phage production. Microb Ecol 35: 235–243.
Kamimura K, Araki M . (1989). Isolation and characterization of a bacteriophage lytic for Desulfovibrio salexigens, a salt-requiring, sulfate-reducing bacterium. Appl Environ Microbiol 55: 645–648.
Kerr RA . (2002). Deep life in the slow, slow lane. Science 296: 1056–1058.
Kutter E, Sulakvelidze A . (2005). Bacteriophages: Biology and Applications. CRC Press: Boca Raton, FL.
Kyle JE, Eydal HSC, Ferris FG, Pedersen K . (2008). Viruses in granitic groundwater from 69 to 450 m depth of the Äspö Hard Rock Laboratory, Sweden. ISME J 2: 571–574.
Laaksoharju M, Tullborg EL, Wikberg P, Wallin B, Smellie J . (1999). Hydrogeochemical conditions and evolution at the Äspö HRL, Sweden. Appl Geochem 14: 835–859.
Lundin A . (2000). Use of firefly luciferase in ATP-related assays of biomass, enzymes, and metabolites. Meth Enzymol 305: 346–370.
Lundin A, Hasenson M, Persson J, Pousette A . (1986). Estimation of biomass in growing cell-lines by adenosine triphosphate assay. Meth Enzymol 133: 27–42.
Middelboe M, Hagstrom A, Blackburn N, Sinn B, Fischer U, Borch NH et al. (2001). Effects of bacteriophages on the population dynamics of four strains of pelagic marine bacteria. Microb Ecol 42: 395–406.
Motamedi M, Pedersen K . (1998). Desulfovibrio aespoeensis sp. nov., a mesophilic sulfate-reducing bacterium from deep groundwater at Äspö hard rock laboratory, Sweden. Int J Syst Bacteriol 48: 311–315.
Noble RT, Fuhrman JA . (1998). Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat Microb Ecol 14: 113–118.
Pedersen K . (2001). Diversity and activity of microorganisms in deep igneous rock aquifers of the Fennoscandian Shield. In: Fredrickson JK, Fletcher M (eds). Subsurface Microgeobiology and Biogeochemistry. Wiley-Liss: New York, pp 97–139.
Pedersen K, Arlinger J, Ekendahl S, Hallbeck L . (1996). 16S rRNA gene diversity of attached and unattached bacteria in boreholes along the access tunnel to the Äspö Hard Rock Laboratory, Sweden. FEMS Microbiol Ecol 19: 249–262.
Pedersen K, Ekendahl S . (1992). Assimilation of CO2 and introduced organic compounds by bacterial communities in ground water from Southeastern Sweden deep crystalline bedrock. Microb Ecol 23: 1–14.
Rapp BJ, Wall JD . (1987). Genetic transfer in Desulfovibrio Desulfuricans. Proc Natl Acad Sci USA 84: 9128–9130.
Suttle CA . (2005). Viruses in the sea. Nature 437: 356–361.
Thingstad TF, Lignell R . (1997). Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat Microb Ecol 13: 19–27.
Walker CB, Stolyar SS, Pinel N, Yen HCB, He ZL, Zhou JZ et al. (2006). Recovery of temperate Desulfovibrio vulgaris bacteriophage using a novel host strain. Environ Microbiol 8: 1950–1959.
Waterbury JB, Valois FW . (1993). Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol 59: 3393–3399.
Weinbauer MG, Peduzzi P . (1994). Frequency, size and distribution of bacteriophages in different marine bacterial morphotypes. Mar Ecol Prog Ser 108: 11–20.
Weinbauer MG, Rassoulzadegan F . (2004). Are viruses driving microbial diversification and diversity? Environ Microbiol 6: 1–11.
Whitman WB, Coleman DC, Wiebe WJ . (1998). Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95: 6578–6583.
Acknowledgements
We wish to thank Johanna Arlinger, Lisa Karlsson and Sara Eriksson at Microbial Analytics Sweden AB for assistance with laboratory cultivations and with sampling at the Äspö HRL, and Jennifer Kyle, Grant Ferris, Mikal Heldal and Gunnar Bratbak for assistance with TEM. We are also grateful for funding from the Swedish Nuclear Fuel and Waste Management Co. and the Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary information
Rights and permissions
About this article
Cite this article
Eydal, H., Jägevall, S., Hermansson, M. et al. Bacteriophage lytic to Desulfovibrio aespoeensis isolated from deep groundwater. ISME J 3, 1139–1147 (2009). https://doi.org/10.1038/ismej.2009.66
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2009.66
Keywords
This article is cited by
-
The Fennoscandian Shield deep terrestrial virosphere suggests slow motion ‘boom and burst’ cycles
Communications Biology (2021)
-
Energy efficiency and biological interactions define the core microbiome of deep oligotrophic groundwater
Nature Communications (2021)
-
Corrosion Inhibition of X80 Steel in Simulated Marine Environment with Marinobacter aquaeolei
Acta Metallurgica Sinica (English Letters) (2019)
-
The biomass and biodiversity of the continental subsurface
Nature Geoscience (2018)
-
The deep continental subsurface: the dark biosphere
International Microbiology (2018)