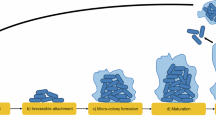
Abstract
Many biofilm populations are known for their exceptional biodiversity, but the relative contributions of the forces that could produce this diversity are poorly understood. This uncertainty grows in the old, well-established communities found on many natural surfaces and in long-term, chronic infections. If the prevailing interactions among species within biofilms are positive, productivity should increase with diversity, but if they tend towards competition or antagonism, productivity should decrease. Here, we describe the parallel evolution of synergistic communities derived from a clone of Burkholderia cenocepacia during ∼1500 generations of biofilm selection. This long-term evolution was enabled by a new experimental method that selects for daily cycles of colonization, biofilm assembly and dispersal. Each of the six replicate biofilm populations underwent a common pattern of adaptive morphological diversification, in which three ecologically distinct morphotypes arose in the same order of succession and persisted. In two focal populations, mixed communities were more productive than any monoculture and each variant benefited from the mixture. These gains in output resulted from asymmetrical cross-feeding between ecotypes and the expansion and partitioning of biofilm space that constructed new niches. Therefore, even in the absence of starting genetic variation, prolonged selection for surface colonization generates a dynamic of ecological succession that enhances productivity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Boles BR, Thoendel M, Singh PK . (2004). Self-generated diversity produces ‘insurance effects’ in biofilm communities. Proc Natl Acad Sci USA 101: 16630–16635.
Brockhurst MA . (2007). Population bottlenecks promote cooperation in bacterial biofilms. PLoS ONE 2: e634.
Brockhurst MA, Hochberg ME, Bell T, Buckling A . (2006). Character displacement promotes cooperation in bacterial biofilms. Curr Biol 16: 2030–2034.
Buckling A, Rainey PB . (2002). The role of parasites in sympatric and allopatric host diversification. Nature 420: 496–499.
Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS et al. (2007). Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci USA 104: 18123–18128.
Chantratita N, Wuthiekanun V, Boonbumrung K, Tiyawisutsri R, Vesaratchavest M, Limmathurotsakul D et al. (2007). Biological relevance of colony morphology and phenotypic switching by Burkholderia pseudomallei. J Bacteriol 189: 807–817.
Connell JH, Slatyer RO . (1977). Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111: 1119–1144.
Costerton JW . (2001). Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol 9: 50–52.
Day T, Young KA . (2004). Competitive and facilitative evolutionary diversification. BioScience 54: 101–109.
Ellis CN, Cooper VS . (2010). Experimental adaptation of Burkholderia cenocepacia to onion medium reduces host range. Appl Environ Microbiol 76: 2387–2396.
Gross K, Cardinale BJ . (2007). Does species richness drive community production or vice versa? Reconciling historical and contemporary paradigms in competitive communities. Am Nat 170: 207–220.
Habets MG, Rozen DE, Hoekstra RF, de Visser JA . (2006). The effect of population structure on the adaptive radiation of microbial populations evolving in spatially structured environments. Ecol Lett 9: 1041–1048.
Hansen SK, Rainey PB, Haagensen JA, Molin S . (2007). Evolution of species interactions in a biofilm community. Nature 445: 533–536.
Harrison F, Buckling A . (2009). Siderophore production and biofilm formation as linked social traits. ISME J 3: 632–634.
Haussler S, Lehmann C, Breselge C, Rohde M, Classen M, Tummler B et al. (2003). Fatal outcome of lung transplantation in cystic fibrosis patients due to small-colony variants of the Burkholderia cepacia complex. Eur J Clin Microbiol Infect Dis 22: 249–253.
Huang LN, De Wever H, Diels L . (2008). Diverse and distinct bacterial communities induced biofilm fouling in membrane bioreactors operated under different conditions. Environ Sci Technol 42: 8360–8366.
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM et al. (1995). 4 New derivatives of the broad-host-range cloning vector Pbbr1mcs, carrying different antibiotic-resistance cassettes. Gene 166: 175–176.
Kuramitsu HK, He X, Lux R, Anderson MH, Shi W . (2007). Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev 71: 653–670.
Lambertsen L, Sternberg C, Molin S . (2004). Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ Microbiol 6: 726–732.
LiPuma JJ, Spilker T, Coenye T, Gonzalez CF . (2002). An epidemic Burkholderia cepacia complex strain identified in soil. Lancet 359: 2002–2003.
Loreau M, Hector A . (2001). Partitioning selection and complementarity in biodiversity experiments. Nature 412: 72–76.
Lyautey E, Jackson C, Cayrou J, Rols J-L, Garabétian F . (2005). Bacterial community succession in natural river biofilm assemblages. Microb Ecol 50: 589–601.
Meyer JR, Kassen R . (2007). The effects of competition and predation on diversification in a model adaptive radiation. Nature 446: 432–435.
Nadell CD, Xavier JB, Foster KR . (2009). The sociobiology of biofilms. FEMS Microbiol Rev 33: 206–224.
Nguyen D, Singh PK . (2006). Evolving stealth: genetic adaptation of Pseudomonas aeruginosa during cystic fibrosis infections. Proc Natl Acad Sci USA 103: 8305–8306.
Nocker A, Lepo JE, Snyder RA . (2004). Influence of an oyster reef on development of the microbial heterotrophic community of an estuarine biofilm. Appl Environ Microbiol 70: 6834–6845.
O’Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, Kolter R . (1999). Genetic approaches to study of biofilms. Methods Enzymol 310: 91–109.
Odling-Smee FJ, Laland KN, Feldman MW . (2003). Niche Construction: The Neglected Process in Evolution, Vol. 37 Princeton University Press: Princeton, NJ.
Odum EP . (1975). Ecology, 2nd edn. Holt, Rinehart, and Winston: New York.
Parke JL, Gurian-Sherman D . (2001). Diversity of the Burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu Rev Phytopathol 39: 225–258.
Ponciano JM, La HJ, Joyce P, Forney LJ . (2009). Evolution of diversity in spatially structured Escherichia coli populations. Appl Environ Microbiol 75: 6047–6054.
Price GR . (1970). Selection and covariance. Nature 227: 520–521.
Rainey PB, Rainey K . (2003). Evolution of cooperation and conflict in experimental bacterial populations. Nature 425: 72–74.
Rainey PB, Travisano M . (1998). Adaptive radiation in a heterogeneous environment. Nature 394: 69–72.
Rakhimova E, Munder A, Wiehlmann L, Bredenbruch F, Tummler B . (2008). Fitness of isogenic colony morphology variants of Pseudomonas aeruginosa in murine airway infection. PLoS ONE 3: e1685.
Ramette A, LiPuma JJ, Tiedje JM . (2005). Species abundance and diversity of Burkholderia cepacia complex in the environment. Appl Environ Microbiol 71: 1193–1201.
Rozen DE, Nadège PJ, de Visser JA, Lenski RE, Schneider D . (2009). Death and cannibalism in a seasonal environment facilitate bacterial coexistence. Ecol Lett 12: 34–44.
Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP . (2000). Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407: 762–764.
Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D’Argenio DA et al. (2006). Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA 103: 8487–8492.
Starkey M, Hickman JH, Ma L, Zhang N, De Long S, Hinz A et al. (2009). Pseudomonas aeruginosa rugose small colony variants have adaptations likely to promote persistence in the cystic fibrosis lung. J Bacteriol 191: 3492–3503; JB.00119-00109.
Stoodley P, Sauer K, Davies DG, Costerton JW . (2002). Biofilms as complex differentiated communities. Annu Rev Microbiol 56: 187–209.
Acknowledgements
We are grateful to R Mooney, M Townley and N Cherim for assistance with imaging; D Hogan, G O’Toole, C Whistler and their laboratories for feedback and N Cooper, L Benton, T Cooper, C Ellis, K Hillesland, P Singh, C Traverse and especially D Rozen, whose comments improved the paper. This research was supported by NIH 1R15AI082528.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Poltak, S., Cooper, V. Ecological succession in long-term experimentally evolved biofilms produces synergistic communities. ISME J 5, 369–378 (2011). https://doi.org/10.1038/ismej.2010.136
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2010.136
Keywords
This article is cited by
-
Low-concentration iron promotes Klebsiella pneumoniae biofilm formation by suppressing succinic acid
BMC Microbiology (2022)
-
Biofilm cultivation facilitates coexistence and adaptive evolution in an industrial bacterial community
npj Biofilms and Microbiomes (2022)
-
Evolutionary transition from a single RNA replicator to a multiple replicator network
Nature Communications (2022)
-
Biofilm antimicrobial susceptibility through an experimental evolutionary lens
npj Biofilms and Microbiomes (2022)
-
Adaptation and phenotypic diversification of Bacillus thuringiensis biofilm are accompanied by fuzzy spreader morphotypes
npj Biofilms and Microbiomes (2022)