Abstract
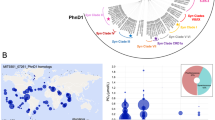
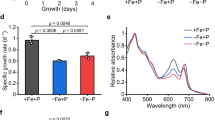
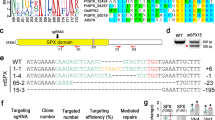
Previous microarray analyses have shown a key role for the two-component system PhoBR (SYNW0947, SYNW0948) in the regulation of P transport and metabolism in the marine cyanobacterium Synechococcus sp. WH8102. However, there is some evidence that another regulator, SYNW1019 (PtrA), probably under the control of PhoBR, is involved in the response to P depletion. PtrA is a member of the cAMP receptor protein transcriptional regulator family that shows homology to NtcA, the global nitrogen regulator in cyanobacteria. To define the role of this regulator, we constructed a mutant by insertional inactivation and compared the physiology of wild-type Synechcococcus sp. WH8102 with the ptrA mutant under P-replete and P-stress conditions. In response to P stress the ptrA mutant failed to upregulate phosphatase activity. Microarrays and quantitative RT-PCR indicate that a subset of the Pho regulon is controlled by PtrA, including two phosphatases, a predicted phytase and a gene of unknown function psip1 (SYNW0165), all of which are highly upregulated during P limitation. Electrophoretic mobility shift assays indicate binding of overexpressed PtrA to promoter sequences upstream of the induced genes. This work suggests a two-tiered response to P depletion in this strain, the first being PhoB-dependent induction of high-affinity PO4 transporters, and the second the PtrA-dependent induction of phosphatases for scavenging organic P. The levels of numerous other transcripts are also directly or indirectly influenced by PtrA, including those involved in cell-surface modification, metal uptake, photosynthesis, stress responses and other metabolic processes, which may indicate a wider role for PtrA in cellular regulation in marine picocyanobacteria.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Aiba H, Mizuno T . (1994). A novel gene whose expression is regulated by the response-regulator, SphR, in response to phosphate limitation in Synechococcus species PCC7942. Mol Microbiol 13: 25–34.
Aiba H, Nagaya M, Mizuno T . (1993). Sensor and regulator proteins from the cyanobacterium Synechococcus species PCC7942 that belong to the bacterial signal-transduction protein families—implication in the adaptive response to phosphate limitation. Mol Microbiol 8: 81–91.
Ammerman JW, Hood RR, Case DA, Cotner JB . (2003). Phosphorus deficiency in the Atlantic: an emerging paradigm in oceanography. EOS 84: 165–170.
Bertilsson S, Berglund O, Karl DM, Chisholm SW . (2003). Elemental composition of marine Prochlorococcus and Synechococcus: implications for the ecological stoichiometry of the sea. Limnol Oceanogr 48: 1721–1731.
Bessey OA, Lowry OH, Brock MJ . (1946). A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem 164: 321–329.
Blindauer CA . (2008). Zinc-handling in cyanobacteria: an update. Chem Biodiv 5: 1990–2013.
Brahamsha B . (1996). A genetic manipulation system for oceanic cyanobacteria of the genus Synechococcus. Appl Environ Microbiol 62: 1747–1751.
Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C et al. (2001). Minimum information about a microarray experiment (MIAME) toward standards for microarray data. Nat Genet 29: 365–371.
Coleman JE . (2003). Structure and mechanism of alkaline phosphatase. Ann Rev Biophys Biomol Struct 21: 441–483.
Cotner JB, Ammerman JW, Peele ER, Bentzen E . (1997). Phosphorus-limited bacterioplankton growth in the Sargasso Sea. Aquat Microb Ecol 13: 141–149.
Dufresne A, Ostrowski M, Scanlan DJ, Garczarek L, Mazard S, Palenik BP et al. (2008). Unraveling the genomic mosaic of a ubiquitous genus of marine cyanobacteria. Genome Biol 9: R90.
Dufresne A, Salanoubat M, Partensky F, Artiguenave F, Axmann IM, Barbe V et al. (2003). Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc Natl Acad Sci USA 100: 10020–10025.
Elledge SJ, Zhou Z, Allen JB . (1992). Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem Sci 17: 119–123.
Heldal M, Scanlan DJ, Norland S, Thingstad F, Mann NH . (2003). Elemental composition of single cells of various strains of marine Prochlorococcus and Synechococcus using X-ray microanalysis. Limnol Oceanogr 48: 1732–1743.
Herrero A, Muro-Pastor AM, Flores E . (2001). Nitrogen control in cyanobacteria. J Bacteriol 183: 411–425.
Hirani TA, Suzuki I, Murata N, Hayashi H, Eaton-Rye JJ . (2001). Characterization of a two-component signal transduction system involved in the induction of alkaline phosphatase under phosphate-limiting conditions in Synechocystis sp. PCC 6803. Plant Mol Biol 45: 133–144.
Itaya K, Ui M . (1966). A new micromethod for the colorimetric determination of inorganic phosphate. Clinica Chimica Acta 14: 361–366.
Karl DM, Tien G . (1997). Temporal variability in dissolved phosphorus concentrations in the subtropical North Pacific Ocean. Mar Chem 56: 77–96.
Korner H, Sofia HJ, Zumft WG . (2003). Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol Rev 27: 559–592.
Lindell D, Padan E, Post AF . (1998). Regulation of ntcA expression and nitrite uptake in the marine Synechococcus sp. strain WH7803. J Bacteriol 180: 1878–1886.
Lohan MC, Statham PJ, Crawford DW . (2002). Total dissolved zinc in the upper water column of the subarctic North East Pacific. Deep-Sea Res Part II 49: 5793–5808.
Luque I, Flores E, Herrero A . (1994). Molecular mechanism for the operation of nitrogen control in cyanobacteria. EMBO J 13: 2862–2869.
Mackey KRM, Paytan A, Grossman AR, Bailey S . (2008). A photosynthetic strategy for coping in a high-light, low-nutrient environment. Limnol Oceanogr 53: 900–913.
Martiny AC, Coleman ML, Chisholm SW . (2006). Phosphate acquisition genes in Prochlorococcus ecotypes: evidence for genome-wide adaptation. Proc Natl Acad Sci USA 103: 12552–12557.
McCarren J, Brahamsha B . (2005). Transposon mutagenesis in a marine Synechococcus strain: isolation of swimming motility mutants. J Bacteriol 187: 4457–4462.
McCarren J, Brahamsha B . (2007). SwmB, a 1.12-megadalton protein that is required for nonflagellar swimming motility in Synechococcus. J Bacteriol 189: 1158–1162.
Moore LR, Ostrowski M, Scanlan DJ, Feren K, Sweetsir T . (2005). Ecotypic variation in phosphorus acquisition mechanisms within marine picocyanobacteria. Aquat Microb Ecol 39: 257–269.
Morel FMM, Rueter JG, Anderson DM, Guillard RRL . (1979). Aquil—chemically defined phytoplankton culture-medium for trace-metal studies. J Phycol 15: 135–141.
Palenik B, Brahamsha B, Larimer FW, Land M, Hauser L, Chain P et al. (2003). The genome of a motile marine Synechococcus. Nature 424: 1037–1042.
Palenik B, Ren QH, Dupont CL, Myers GS, Heidelberg JF, Badger JH et al. (2006). Genome sequence of Synechococcus CC9311: insights into adaptation to a coastal environment. Proc Natl Acad Sci USA 103: 13555–13559.
Partensky F, Hess WR, Vaulot D . (1999). Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev 63: 106–127.
Salgado H, Gama-Castro S, Martinez-Antonio A, Diaz-Peredo E, Sanchez-Solano F, Peralta-Gil M et al. (2004). RegulonDB (version 4.0): transcriptional regulation, operon organization and growth conditions in Escherichia coli K-12. Nucleic Acids Res 32: D303–D306.
Scanlan DJ, Bourne JA, Mann NH . (1997a). A putative transcriptional activator of the Crp/Fnr family from the marine cyanobacterium Synechococcus sp WH7803. J Appl Phycol 8: 565–567.
Scanlan DJ, Mann NH, Carr NG . (1993). The response of the picoplanktonic marine cyanobacterium Synechococcus species WH7803 to phosphate starvation involves a protein homologous to the periplasmic phosphate-binding protein of Escherichia coli. Mol Microbiol 10: 181–191.
Scanlan DJ, Silman NJ, Donald KM, Wilson WH, Carr NG, Joint I et al. (1997b). An immunological approach to detect phosphate stress in populations and single cells of photosynthetic picoplankton. Appl Environ Microbiol 63: 2411–2420.
Scanlan DJ, Ostrowski M, Mazard S, Dufresne A, Garczarek L, Hess WR et al. (2009). Ecological genomics of marine picocyanobacteria. Microbiol Mol Biol Rev 73: 249–299.
Snyder DS, Brahamsha B, Azadi P, Palenik B . (2009). Structure of compositionally simple lipopolysaccharide from marine Synechococcus. J Bacteriol 191: 5499–5509.
Silberbach M, Hüser A, Kalinowski J, Pühler A, Walter B, Kramer R et al. (2005). DNA microarray analysis of the nitrogen starvation response of Corynebacterium glutamicum. J Biotech 119: 357–367.
Su ZC, Dam P, Chen X, Olman V, Jiang T, Palenik B et al. (2003). Computational inference of regulatory pathways in microbes. An application to phosphorus assimilation pathways in Synechococcus WH8102. Genome Inform 14: 3–13.
Su ZC, Olman V, Mao FL, Xu Y . (2005). Comparative genomics analysis of NtcA regulons in cyanobacteria: regulation of nitrogen assimilation and its coupling to photosynthesis. Nucleic Acids Res 33: 5156–5171.
Su ZC, Olman V, Xu Y . (2007). Computational prediction of Pho regulons in cyanobacteria. BMC Genomics 8: 156.
Suzuki S, Ferjani A, Suzuki I, Murata N . (2004). The SphS–SphR two component system is the exclusive sensor for the induction of gene expression in response to phosphate limitation in Synechocystis. J Biol Chem 279: 13234–13240.
Tai V, Paulsen IT, Phillippy K, Johnson DA, Palenik B . (2009). Whole-genome microarray analyses of Synechococcus–Vibrio interactions. Environ Microbiol 11: 2698–2709.
Tetu SG, Brahamsha B, Johnson DA, Tai V, Phillippy K, Palenik B et al. (2009). Microarray analysis of phosphate regulation in the marine cyanobacterium Synechococcus sp WH8102. ISME J 3: 835–849.
Thingstad TF, Krom MD, Mantoura RFC, Flaten GAF, Groom S, Herut B et al. (2005). Nature of phosphorus limitation in the ultraoligotrophic eastern Mediterranean. Science 309: 1068–1071.
Tusher VG, Tibshirani R, Chu G . (2001). Significance Analysis of Microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121.
Van Mooy BAS, Rocap G, Fredricks HF, Evans CT, Devol AH . (2006). Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligotrophic marine environments. Proc Natl Acad Sci USA 103: 8607–8612.
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA et al. (2004). Environmental genome shotgun sequencing of the Sargasso Sea. Science 304: 66–74.
Waterbury JB, Willey JM . (1988). Isolation and growth of marine planktonic cyanobacteria. Methods Enzymol 167: 100–105.
Zubkov MV, Mary I, Woodward EMS, Warwick PE, Fuchs BM, Scanlan DJ et al. (2007). Microbial control of phosphate in the nutrient-depleted North Atlantic subtropical gyre. Environ Microbiol 9: 2079–2089.
Zwirglmaier K, Jardillier L, Ostrowski M, Mazard S, Garczarek L, Vaulot D et al. (2008). Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages among oceanic biomes. Environ Microbiol 10: 147–161.
Acknowledgements
We thank Bianca Brahamsha for assistance and guidance in preparing the ptrA gene knockout and for supplying axenic cultures of Synechococcus sp. WH8102. MO and SM were supported by the EU FP5 Program Margenes (QLRT-2001-0226), the FP6 EU Marine Genomics Network and NERC Grants NE/C0005361/1, NE/F004249/1 and NE/D003385/1 to DJS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Ostrowski, M., Mazard, S., Tetu, S. et al. PtrA is required for coordinate regulation of gene expression during phosphate stress in a marine Synechococcus. ISME J 4, 908–921 (2010). https://doi.org/10.1038/ismej.2010.24
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2010.24