Abstract
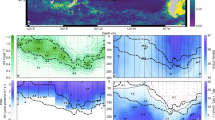
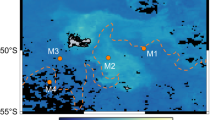
Global estimates indicate the oceans are responsible for approximately half of the carbon dioxide fixed on Earth. Organisms ⩽5 μm in size dominate open ocean phytoplankton communities in terms of abundance and CO2 fixation, with the cyanobacterial genera Prochlorococcus and Synechococcus numerically the most abundant and more extensively studied compared with small eukaryotes. However, the contribution of specific taxonomic groups to marine CO2 fixation is still poorly known. In this study, we show that among the phytoplankton, small eukaryotes contribute significantly to CO2 fixation (44%) because of their larger cell volume and thereby higher cell-specific CO2 fixation rates. Within the eukaryotes, two groups, herein called Euk-A and Euk-B, were distinguished based on their flow cytometric signature. Euk-A, the most abundant group, contained cells 1.8±0.1 μm in size while Euk-B was the least abundant but cells were larger (2.8±0.2 μm). The Euk-B group comprising prymnesiophytes (73±13%) belonging largely to lineages with no close cultured counterparts accounted for up to 38% of the total primary production in the subtropical and tropical northeast Atlantic Ocean, suggesting a key role of this group in oceanic CO2 fixation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Amann R, Fuchs BM . (2008). Single–cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nature Rev 6: 339–348.
Andersen RA . (2004). Biology and systematics of heterokont and haptophyte algae. Am J Bot 91: 1508–1522.
Behrenfeld MJ, Randerson JT, McClain CR, Feldman GC, Los SO, Tucker CJ et al. (2001). Biospheric primary production during an ENSO transition. Science 291: 2594–2597.
Bidigare RR, Ondrusek ME . (1996). Spatial and temporal variability of phytoplankton pigment distributions in the central equatorial Pacific Ocean. Deep–Sea Res II 43: 809–833.
Biegala IC, Not F, Vaulot D, Simon N . (2003). Quantitative assessment of picoeukaryotes in the natural environment by using taxon-specific oligonucleotide probes in association with tyramide signal amplification-fluorescence in situ hybridization and flow cytometry. Appl Environ Microbiol 69: 5519–5529.
Booth BC, Marchant HJ . (1987). Parmales, a new order of marine chrysophytes, with descriptions of three new genera and seven new species. J Phycol 23: 245–260.
Bravo–Sierra E, Hernández–Becerril DU . (2003). Parmales (Chrysophyceae) from the Gulf of Tehuantepec, Mexico, including the description of a new species, Tetraparma insecta sp nov, and a proposal to the taxonomy of the group. J Phycol 39: 577–583.
Díez B, Pedrós–Alío C, Massana R . (2001). Study of genetic diversity of eukaryotic picoplankton in different oceanic regions by small–subunit rRNA gene cloning and sequencing. Appl Environ Microbiol 67: 2932–2941.
Foulon E, Not F, Jalabert F, Cariou T, Massana R, Simon N . (2008). Ecological niche partitioning in the picoplanktonic green alga Micromonas pusilla: evidence from environmental surveys using phylogenetic probes. Environ Microbiol 10: 2433–2443.
Fuller NJ, Campbell C, Allen DJ, Pitt FD, Zwirglmaier K, Le Gall F et al. (2006a). Analysis of photosynthetic picoeukaryote diversity at open ocean sites in the Arabian Sea using a PCR biased towards marine algal plastids. Aquat Microb Ecol 43: 79–93.
Fuller NJ, Marie D, Partensky F, Vaulot D, Post AF, Scanlan DJ . (2003). Clade–specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl Environ Microbiol 69: 2430–2443.
Fuller NJ, Tarran GA, Cummings DG, Woodward EMS, Orcutt KM, Yallop M et al. (2006b). Molecular analysis of photosynthetic picoeukaryote community structure along an Arabian Sea transect. Limnol Oceanogr 51: 2502–2514.
Giovannoni SJ, Delong EF, Olsen GJ, Pace NR . (1988). Phylogenetic group–specific oligonucleotide probes for identification of single microbial cells. J Bacteriol 170: 720–726.
Goericke R . (1998). Response of phytoplankton community structure and taxon-specific growth rates to seasonally varying physical forcing in the Sargasso Sea off Bermuda. Limnol Oceanogr 43: 921–935.
Grob C, Ulloa O, Claustre H, Huot Y, Alarcón G, Marie D . (2007). Contribution of picoplankton to the total particulate organic carbon (POC) concentration in the eastern South Pacific. Biogeosci Discuss 4: 1–37.
Irwin B . (1991). Coulometric measurement of primary production, with comparison against dissolved oxygen and 14C methods in a seasonal study. Mar Ecol Prog Ser 71: 97–102.
Jones HLJ, Durjun P, Leadbeater BSC, Green JC . (1995). The relationship between photoacclimation and phagotrophy with respect to chlorophyll a, carbon and nitrogen content, and cell size of Chrysochromulina brevifilum (Prymnesiophyceae). Phycologia 34: 128–134.
Kawachi M, Inouye I, Maeda O, Chihara M . (1991). The haptonema as a food-capturing device: observations on Chrysochromulina hirta (Prymnesiophyceae). Phycologia 30: 563–573.
Landry MR, Constantinou J, Latasa M, Brown SL, Bidigare RR, Ondrusek ME . (2000). Biological response to iron fertilization in the eastern equatorial Pacific (IronEx II). III. Dynamics of phytoplankton growth and microzooplankton grazing. Mar Ecol Prog Ser 201: 57–72.
Larsen A, Tanaka T, Zubkov MV, Thingstad TF . (2008). P-affinity measurements of specific osmotroph populations using cell-sorting flow cytomtery. Limnol Oceanogr Meth 6: 355–363.
Latasa M, Bidigare RR . (1998). A comparison of phytoplankton populations of the Arabian Sea during the spring intermonsoon and southwest monsoon of 1995 as described by HPLC-analyzed pigments. Deep Sea Res Pt II 45: 2133–2170.
Lepère C, Vaulot D, Scanlan DJ . (2009). Photosynthetic picoeukaryote community structure in the south east Pacific Ocean encompassing the most oligotrophic waters on Earth. Environ Microbiol 11: 3105–3117.
Li WKW . (1994). Primary productivity of prochlorophytes, cyanobacteria, and eucaryotic ultraphytoplankton: measurements from flow cytometric sorting. Limnol Oceanogr 39: 169–175.
Liu H, Aris-Brosou S, Probert I, de Vargas C . (2010). A timeline of the environmental genetics of the haptophytes. Mol Biol Evol 27: 161–176.
Liu H, Probert I, Uitz J, Claustre H, Aris-Brosou S, Frada M et al. (2009). Extreme diversity in noncalcifying haptophytes explains a major pigment paradox in open oceans. Proc Natl Acad Sci USA 106: 12803–12808.
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar et al. (2004). ARB: a software environment for sequence data. Nucl Acids Res 32: 1363–1371.
Marañón E, Holligan PM, Barciela R, Gonzalez N, Mourino B, Pazo MJ et al. (2001). Patterns of phytoplankton size structure and productivity in contrasting open-ocean environments. Mar Ecol Prog Ser 216: 43–56.
Marañón E, Holligan PM, Varela M, Mouriño B, Bale AJ . (2000). Basin–scale variability of phytoplankton biomass, production and growth in the Atlantic Ocean. Deep–Sea Res I 47: 825–857.
Marie D, Zhu F, Balagúe V, Ras J, Vaulot D . (2006). Eukaryotic picoplankton communities of the Mediterranean Sea in summer assessed by molecular approaches (DGGE, TTGE, QPCR). FEMS Microbiol Ecol 55: 403–415.
McDonald SM, Sarno D, Scanlan DJ, Zingone A . (2007). Genetic diversity of eukaryotic ultraphytoplankton in the Gulf of Naples during an annual cycle. Aquat Microb Ecol 50: 75–89.
Moon–van der Staay SY, De Wachter R, Vaulot D . (2001). Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 409: 607–610.
Moon-van der Staay SY, van der Staay GWM, Guillou L, Vaulot D . (2000). Abundance and diversity of prymnesiophytes in the picoplankton community from the equatorial Pacific Ocean inferred from 18S rDNA sequences. Limnol Oceanogr 45: 98–109.
Morán XAG . (2007). Annual cycle of picophytoplankton photosynthesis and growth rates in a temperate coastal ecosystem: a major contribution to carbon fluxes. Aquat Microb Ecol 49: 267–279.
Neufeld JD, Vohra J, Dumont MG, Lueders T, Manefield M, Friedrich MW et al. (2007). DNA stable–isotope probing. Nat Methods 2: 860–866.
Not F, Latasa M, Marie D, Cariou T, Vaulot D, Simon N . (2004). A single species Micromonas pusilla (Prasinophyceae) dominates the eukaryotic picoplankton in the western English Channel. Appl Environ Microbiol 70: 4064–4072.
Not F, Latasa M, Scharek R, Viprey M, Karleskind P, Balague V et al. (2008). Protistan assemblages across the Indian Ocean, with a specific emphasis on the picoeukaryotes. Deep-Sea Res I 55: 1456–1473.
Not F, Simon N, Biegala IC, Vaulot D . (2002). Application of fluorescent in situ hybridization coupled with tyramide signal amplification (FISH-TSA) to assess eukaryotic picoplankton composition. Aquat Microb Ecol 28: 157–166.
Novarino G, Mills DK, Hannah F . (1997). Pelagic flagellate populations in the southern North Sea, 1988–89. I. Qualitative observations. J Plankton Res 19: 1081–1109.
Pérez V, Fernández E, Marañón E, Morán XAG, Zubkov MV . (2006). Vertical distribution of phytoplankton biomass, production and growth in the Atlantic subtropical gyres. Deep–Sea Res I 53: 1616–1634.
Poulton AJ, Holligan PM, Hickman A, Kim YN, Adey TR, Stinchcombe MC et al. (2006). Phytoplankton carbon fixation, chlorophyll–biomass and diagnostic pigments in the Atlantic Ocean. Deep–Sea Res II 53: 1593–1610.
Raven JA . (1998). The twelfth tansley lecture. Small is beautiful: the picophytoplankton. Functional Ecol 12: 503–513.
Richardson TL, Jackson GA . (2007). Small phytoplankton and carbon export from the surface ocean. Science 315: 838–840.
Romari K, Vaulot D . (2004). Composition and temporal variability of picoeukaryote communities at a coastal site of the English Channel from 18S rDNA sequences. Limnol Oceanogr 49: 784–798.
Schönhuber W, Fuchs B, Juretschko S, Amann R . (1997). Improved sensitivity of whole–cell hybridization by the combination of horseradish peroxidase–labeled oligonucleotides and tyramide signal amplification. Appl Environ Microbiol 63: 3268–3273.
Shi XL, Marie D, Jardillier L, Scanlan DJ, Vaulot D . (2009). Groups without cultured representatives dominate eukaryotic picophytoplankton in the oligotrophic south east Pacific Ocean. PLoS One 4: e7657.
Simon N, Campbell L, Ornolfsdottir E, Groben R, Guillou L, Lange M et al. (2000). Oligonucleotide probes for the identification of three algal groups by dot blot and fluorescent whole–cell hybridization. J Euk Microbiol 47: 76–84.
Simon N, Lebot N, Marie D, Partensky F, Vaulot D . (1995). Fluorescent in situ hybridization with rRNA–targeted oligonucleotide probes to identify small phytoplankton by flow cytometry. Appl Environ Microbiol 61: 2506–2513.
Steemann-Nielsen E . (1952). The use of radioactive carbon (14C) for measuring organic production in the sea. J Cons Int Explor Mer 18: 117–140.
Teira E, Mourino B, Marañón E, Perez V, Pazo MJ, Serret P et al. (2005). Variability of chlorophyll and primary production in the Eastern North Atlantic Subtropical Gyre: potential factors affecting phytoplankton activity. Deep–Sea Res I 52: 569–588.
Throndsen J . (1996). The planktonic marine flagellates. In: Tomas CR (ed). Identifying Marine Phytoplankton. Academic Press: San Diego, pp 591–730.
Urein F, Massana R, Alonso-Sáez L, Gasol JM . (2007). Significant year-round effect of small mixotrophic flagellates on bacterioplankton in an oligotrophic coastal system. Limnol Oceanogr 52: 456–469.
Vaulot D, Eikrem W, Viprey M, Moreau H . (2008). The diversity of small eukaryotic phytoplankton (<3 μm) in marine ecosystems. FEMS Microbiol Rev 32: 795–820.
Viprey M, Guillou L, Ferréol M, Vaulot D . (2008). Wide genetic diversity of picoplanktonic green algae (Chloroplastidia) in the Mediterranean Sea uncovered by a phylum-biased PCR approach. Environ Microbiol 10: 1804–1822.
West NJ, Schönhuber WA, Fuller NJ, Amann RI, Rippka R, Post AF et al. (2001). Closely related Prochlorococcus genotypes show remarkably different depth distributions in two oceanic regions as revealed by in situ hybridisation using 16S rRNA–targeted oligonucleotides. Microbiology 147: 1731–1744.
Wilmotte A, Demonceau C, Goffart A, Hecq JH, Demoulin V, Crossley AC . (2002). Molecular and pigment studies of the picophytoplankton in a region of the Southern Ocean (42–541S, 141–1441E) in March 1998. Deep–Sea Res II 49: 3351–3363.
Worden AZ, Nolan JK, Palenik B . (2004). Assessing the dynamics and ecology of marine picophytoplankton: the importance of the eukaryotic component. Limnol Oceanogr 49: 168–179.
Worden AZ, Not F . (2008). Ecology and diversity of picoeukaryotes. In: Kirchman DL (ed). Microbial Ecology of the Ocean. John Wiley & Sons, Inc.: New York, pp 159–196.
Wright SW, Ishikawa A, Marchant HJ, Davidson AT, van den Enden RL, Nash GV . (2009). Composition and significance of picophytoplankton in Antarctic waters. Polar Biol 32: 797–808.
Young JR, Geisen M, Probert I . (2005). A review of selected aspects of coccolithophore biology with implications for paleobiodiversity estimation. Micropaleontology 51: 267–288.
Zubkov MV . (2009). Photoheterotrophy in marine prokaryotes. J Plankton Res 31: 933–938.
Zubkov MV, Sleigh MA, Burkill PH, Leakey RJG . (2000). Picoplankton community structure on the Atlantic Meridional Transect: a comparison between seasons. Prog Oceanogr 45: 369–386.
Zubkov MV, Burkill PH . (2006). Syringe pumped high speed flow cytometry of oceanic phytoplankton. Cytometry Part A 69A: 1010–1019.
Zubkov MV, Burkill PH, Topping JN . (2007). Flow cytometric enumeration of DNA stained oceanic planktonic protists. J Plankton Res 29: 79–86.
Zubkov MV, Leakey RJ . (2009). Evaluation of the efficiency of metabolism of dinoflagellate phosphorus and carbon by a planktonic ciliate. Eur J Protist 45: 166–173.
Zubkov MV, Tarran GA . (2008). High bacterivory by the smallest phytoplankton in the North Atlantic Ocean. Nature 455: 224–227.
Acknowledgements
We gratefully acknowledge the captain, officers and crew aboard the RRS Discovery for their help during the cruise. We thank Eric Achterberg and other scientists, involved in the Natural Environment Research Council (NERC) UK Surface Ocean Lower Atmosphere Study (SOLAS) project NE/C001931/1, for an opportunity given to LJ to participate in the cruise. We also thank I Mary for advice on the cell concentrating procedure, W Gaze for sharing microscope facilities, F LeGall and D Vaulot for sharing cultures of the Roscoff Culture Collection and L Lehtovirta and M Nicoll for optimizing the DNA extraction protocol from sorted cells. This study was supported by NERC grant NE/C003160/1 and a NERC funded PhD studentship to JP.Authors contribution: DJS and MZ designed the study. Tracer work, FISH counts and data analysis was carried out by LJ. Flow cytometry work was performed by MVZ on board and by LJ ashore. JP and LJ constructed the genetic libraries. LJ, MVZ and DJS wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Jardillier, L., Zubkov, M., Pearman, J. et al. Significant CO2 fixation by small prymnesiophytes in the subtropical and tropical northeast Atlantic Ocean. ISME J 4, 1180–1192 (2010). https://doi.org/10.1038/ismej.2010.36
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2010.36
Keywords
This article is cited by
-
Elucidating the picocyanobacteria salinity divide through ecogenomics of new freshwater isolates
BMC Biology (2022)
-
Novel functional insights into a modified sugar-binding protein from Synechococcus MITS9220
Scientific Reports (2022)
-
Niche partitioning by photosynthetic plankton as a driver of CO2-fixation across the oligotrophic South Pacific Subtropical Ocean
The ISME Journal (2022)
-
Small phytoplankton contribute greatly to CO2-fixation after the diatom bloom in the Southern Ocean
The ISME Journal (2021)
-
Pili allow dominant marine cyanobacteria to avoid sinking and evade predation
Nature Communications (2021)