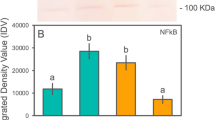
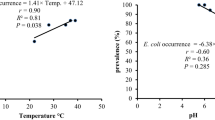
Abstract
Sea surface temperatures (SST) are rising because of global climate change. As a result, pathogenic Vibrio species that infect humans and marine organisms during warmer summer months are of growing concern. Coral reefs, in particular, are already experiencing unprecedented degradation worldwide due in part to infectious disease outbreaks and bleaching episodes that are exacerbated by increasing SST. For example, Vibrio coralliilyticus, a globally distributed bacterium associated with multiple coral diseases, infects corals at temperatures above 27 °C. The mechanisms underlying this temperature-dependent pathogenicity, however, are unknown. In this study, we identify potential virulence mechanisms using whole genome sequencing of V. coralliilyticus ATCC (American Type Culture Collection) BAA-450. Furthermore, we demonstrate direct temperature regulation of numerous virulence factors using proteomic analysis and bioassays. Virulence factors involved in motility, host degradation, secretion, antimicrobial resistance and transcriptional regulation are upregulated at the higher virulent temperature of 27 °C, concurrent with phenotypic changes in motility, antibiotic resistance, hemolysis, cytotoxicity and bioluminescence. These results provide evidence that temperature regulates multiple virulence mechanisms in V. coralliilyticus, independent of abundance. The ecological and biological significance of this temperature-dependent virulence response is reinforced by climate change models that predict tropical SST to consistently exceed 27 °C during the spring, summer and fall seasons. We propose V. coralliilyticus as a model Gram-negative bacterium to study temperature-dependent pathogenicity in Vibrio-related diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Alves N, Neto OSM, Silva BSO, de Moura RL, Francini-Filho RB, Castro CB et al. (2010). Diversity and pathogenic potential of vibrios isolated from Abrolhos Bank corals. Environ Microbiol Rep 2: 90–95.
Aronson RB, Precht WF, Macintyre IG, Murdoch TJ . (2000). Coral bleach-out in Belize. Nature 405: 36.
Arotsker L, Siboni N, Ben-Dov E, Kramarsky-Winter E, Loya Y, Kushmaro A . (2009). Vibrio sp. as a potentially important member of the black band disease (BBD) consortium in Favia sp. corals. FEMS Microbiol Ecol 70: 515–524.
Austin B, Austin D, Sutherland R, Thompson FL, Swings J . (2005). Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ Microbiol 7: 1488–1495.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA et al. (2008). The RAST server: rapid annotations using subsystems technology. BMC Genomics 9: 1–15.
Bassler BL, Wright M, Silverman MR . (1994). Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol Microbiol 13: 273–286.
Ben-Haim Y, Thompson FL, Thompson CC, Cnockaert MC, Hoste B, Swings J et al. (2003a). Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Internatl J Syst Evol Microbiol 53: 309–315.
Ben-Haim Y, Zicherman-Keren M, Rosenberg E . (2003b). Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Environ Microbiol 69: 4236–4242.
Bina XR, Provenzano D, Nguyen N, Bina JE . (2008). Vibrio cholerae RND family efflux systems are required for antimicrobial resistance, optimal virulence factor production, and colonization of the infant mouse small intestine. Infect Immun 76: 3595–3605.
Bonemann G, Pietrosiuk A, Diemand A, Zentgraf H, Mogk A . (2009). Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J 28: 315–325.
Carpenter KE, Abrar M, Aeby G, Aronson R, Banks S, Bruckner AW et al. (2008). One-third of reef-building corals face extinction risk from climate change and local impacts. Science 321: 560–563.
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ . (2008). The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 6: 17–28.
Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ et al. (2009). Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci USA 106: 15442–15447.
de Oliveira Santos E, Alves Jr N, Dias GM, Mazotto AM, Vermelho A, Vora GJ et al. (2011). Genomic and proteomic analyses of the coral pathogen Vibrio coralliilyticus reveal a diverse virulence repertoire. ISME J 5: 1471–1483.
Donner SD, Knutson TR, Oppenheimer M . (2007). Model-based assessment of the role of human-induced climate change in the 2005 Caribbean coral bleaching event. Proc Natl Acad Sci USA 104: 5483–5488.
Dunn SR . (2009). Immunorecognition and immunoreceptors in the Cnidaria. Invertebr Surv J 6: 7–14.
Eakin CM, Morgan JA, Heron SF, Smith TB, Liu G, Alvarez-Filip L et al. (2010). Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS One 5: e13969.
Efrony R, Atad I, Rosenberg E . (2009). Phage therapy of coral white plague disease: properties of phage BA3. Curr Microbiol 58: 139–145.
Eng JK, McCormack AL, Yates JR . (1994). An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5: 976–989.
Fernandez MAL, Bauernfeind A, Jimenez JD, Gil CL, Omeiri NE, Guibert DH . (2009). Influence of temperature and rainfall on the evolution of cholera epidemics in Lusaka, Zambia, 2003–2006: analysis of a time series. Trans R Soc Trop Med Hyg 103: 137–143.
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM . (2007). DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57: 81–91.
Harvell CD, Altizer S, Cattadori IM, Harrington L, Weil E . (2009). Climate change and wildlife diseases: when does the host matter the most? Ecology 90: 912–920.
Hasegawa H, Chatterjee A, Cui Y, Chatterjee AK . (2005). Elevated temperature enhances virulence of Erwinia carotovora subsp. carotovora strain EC153 to plants and stimulates production of the quorum sensing signal, N-acyl homoserine lactone, and extracellular proteins. Appl Environ Microbiol 71: 4655–4663.
Hashizume M, Faruque ASG, Terao T, Yunus M, Streatfield K, Yamamoto T et al. (2011). The Indian Ocean dipole and cholera incidence in Bangladesh: a time series. Environ Health Perspect 119: 239–244.
Hendrickson EL, Xia Q, Wang T, Leigh JA, Hackett M . (2006). Comparison of spectral counting and metabolic stable isotope labeling for use with quantitative microbial proteomics. Analyst 131: 1335–1341.
Hoegh-Guldberg O, Bruno JF . (2010). The impact of climate change on the world's marine ecosystems. Science 328: 1523–1528.
Hughes DT, Sperandio V . (2008). Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6: 111–120.
Igbinosa EO, Okoh AI . (2008). Emerging Vibrio species: an unending threat to public health in developing countries. Res Microbiol 159: 495–506.
IPCC (2007). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press: Cambridge, UK.
Iwamoto M, Ayers T, Mahon BE, Swerdlow DL . (2010). Epidemiology of seafood-associated infections in the United States. Clin Microbiol Rev 23: 399–411.
Jeffries VE . (1982). Three Vibrio strains pathogenic to larvae of Crassostrea gigas and Ostrea edulis. Aquaculture 29: 201–226.
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L . (2009). Two pathogens of Greenshell (TM) mussel larvae, Perna canaliculus: Vibrio splendidus and a V.coralliilyticus/neptunius-like isolate. J Fish Dis 32: 499–507.
Koenig JE, Bourne DG, Curtis B, Dlutek M, Stokes HW, Doolittle WF et al. (2011). Coral-mucus-associated Vibrio integrons in the Great Barrier Reef: genomic hotspots for environmental adaptation. ISME J 5: 962–972.
Latour X, Diallo S, Chevalier S, Morin D, Smadja B, Burini J et al. (2007). Thermoregulation of N-acyl homoserine lactone-based quorum sensing in the soft rot bacterium Pectobacterium atrosepticum. Appl Environ Microbiol 73: 4078–4081.
Lee J, Rho JB, Park KS, Kim CB, Han Y, Choi SH et al. (2004). Role of flagellum and motility in pathogenesis of Vibrio vulnificus. Infect Immun 72: 4905–4910.
Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL . (2004). The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118: 69–82.
Li H, Granat A, Stewart V, Gillespie JR . (2008). RpoS, H-NS, and DsrA inluence EHEC hemolysin operon(ehxCABD) transcription in Escherichia coli O157:H7 strainEDL933. FEMS Microbiol Lett 285: 257–262.
Liu MC, Naka H, Crosa JH . (2009). HlyU acts as an H-NS antirepressor in the regulation of the RTX toxin gene essential for the virulence of the human pathogen Vibrio vulnificus CMCP6. Mol Microbiol 72: 491–505.
Lo H, Lin J, Chen Y, Chen C, Shao C, Lai Y et al. (2011). RTX toxin enhances the survival of Vibrio vulnificus during infection by protecting the organism from phagocytosis. J Infect Dis 203: 1866–1874.
Makino K, Oshima K, Kurokawa K, Katsushi Y, Honda T, Shinagawa H et al. (2003). Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from V. cholerae. Lancet 361: 743–749.
Meron D, Efrony R, Johnson WR, Schaefer AL, Morris PJ, Rosenberg E et al. (2009). Role of flagella in virulence of the coral pathogen Vibrio coralliilyticus. Appl Environ Microbiol 75: 5704–5707.
Millikan DS, Ruby E . (2004). Vibrio fischeri Flagellin A is essential for normal motility and for symbiotic competence during initial squid light organ colonization. J Bacteriol 186: 4315–4325.
Ng W, Bassler BL . (2009). Bacterial quorum-sensing network architectures. Annu Rev Genet 43: 197–222.
NOAA (2011). National Climatic Data Center, State of the climate: global analysis annual 2010, published online January 2011, retrieved on 20 January 2011 from http://www.ncdc.noaa.gov/sotc/gloabl/.
Oh MH, Lee SM, Lee DH, Choi SH . (2009). Regulation of the Vibrio vulnificus hupA gene by temperature alteration and cyclic AMP receptor protein and evaluation of its role in virulence. Infect Immun 77: 1208–1215.
Okada K, Iida T, Kita-Tsukamotot K, Honda T . (2005). Vibrios commonly possess two chromosomes. J Bacteriol 187: 752–757.
Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR et al. (2005). Comparison of lable-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics 4: 1487–1502.
Olivier V, Haines K, Tan Y, Satchell KJF . (2007). Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor 01 strains. Infect Immun 75: 5035–5042.
Ormonde P, Horstedt P, O’toole R, Milton D . (2000). Role of motility in adherence to and invasion of a fish cell line by Vibrio anguillarum. J Bacteriol 182: 2326–2328.
Overbeek R, Begley T, Butler RM, Choudhuri JV, Chuang HY, Cohoon M et al. (2005). The subsystems approach to genome annotation and its use in the project to annotate 1000 genomes. Nucleic Acids Res 33: 5691–5702.
Parkinson JS . (2004). Signal amplification in bacterial chemotaxis through receptor teamwork. ASM News 70: 575–582.
Patz JA, Campbell-Lendrum D, Holloway T, Foley JA . (2005). Impact of regional climate change on human health. Nature 438: 310–317.
Piddock LJV . (2006). Multidrug-resistance efflux pumps—not just for resistance. Nat Rev Microbiol 4: 629–636.
Pollock JF, Wilson B, Johnson WR, Morris PJ, Willis BL, Bourne DG . (2010). Phylogeny of the coral pathogen Vibrio coralliilyticus. Environ Microbiol Rep 2: 172–178.
Rosenberg E, Kushmaro A . (2011). Microbial diseases of corals: pathology and physiology. In: Dubinsky Z, Stambler N (eds). Coral Reefs: An Ecosystem In Transition. Springer: New York, pp 451–464.
Smith G, Hayasaka SS . (1982). Nitrogenase activity associated with Halodule wrightii roots. Appl Environ Microbiol 43: 1244–1248.
Stonehouse EA, Hulbert RR, Nye MB, Skorupski K, Taylor RK . (2011). H-NS binding and repression of the ctx promoter in Vibrio cholerae. J Bacteriol 193: 979–988.
Sussman M, Mieog JC, Doyle J, Victor S, Willis B, Bourne DG . (2009). Vibrio zinc-metalloprotease causes photoinactivation of coral endosymbionts and coral tissue lesions. PLoS Biol 4: e4511.
Sussman M, Willis B, Victor S, Bourne D . (2008). Coral pathogens identified for white syndrome (WS) epizootics in the Indo-Pacific. PLoS One 3: e2393.
Tait K, Hutchinson Z, Thompson FL, Munn CB . (2010). Quorum sensing signal production and inhibition bt coral-associated vibrios. Environ Microbiol Rep 2: 145–150.
Thompson FL, Iida T, Swings J . (2004). Biodiversity of vibrios. Microbiol Mol Biol Rev 68: 403–431.
Tran HT, Krushkal J, Antommattei FM, Lovley DR, Weis RM . (2008). Comparative genomics of Geobacter chemotaxis genes reveals diverse signaling function. BCM Genomics 9: 1–15.
Vezzulli L, Previati M, Pruzzo C, Marchese A, Bourne DG, Cerrano C et al. (2010). Vibrio infections triggering mass mortality events in a warming Mediterranean Sea. Environ Microbiol 12: 2007–2019.
Vidal-Dupiol J, Ladriere O, Meistertzheim A, Foure L, Adjeroud M, Mitta G . (2011). Physiological responses of the scleractinian coral Pocillopora damicornis to bacterial stress from Vibrio coralliilyticus. J Experimental Biol 214: 1533–1545.
Vizcaino MI, Johnson WR, Kimes NE, Williams K, Torralba M, Nelson KE et al. (2010). Antimicrobial resistance of the coral pathogen Vibrio coralliilyticus and Caribbean sister phylotypes isolated from a diseased octocoral. Microb Ecol 59: 646–657.
Whiteman E . (2010). A fatal switch for corals? PLoS Biol 8: e1000346.
Wooldridge K . (2009). Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis. Caister Academic Press: Portland.
Acknowledgements
This work was supported by NSF Biodiversity Surveys and Inventories (DEB 0516347, DEB 0964997) to PJM, a NSF Foundation Graduate Research Fellowship to NEK, the NOAA OHHI Distinguished Scholars program to RCC, and NOAA (SO660009) and NIH (1R01A139129-01) to RRC. Sequencing support was received from the Office of the Chief Scientist (USA), University of Maryland Vibrio Genome Sequencing Project and the Los Alamos National Laboratory. The Fellowship for Interpretation of Genomes (FIG, Argonne National Laboratory) and the National Institute of Allergy and Infectious Diseases (NIH) were instrumental in supporting the RAST and the SEED data analysis environments. We thank Veronika Vonstein and Ross Overbeek for their assistance with the RAST system, Lisa Kilpatrick (NIST) and Kevin Schey/Jennifer Bethard (MUSC Mass Spectrometry Facility) for the use of their facilities to perform the two-dimensional liquid chromatography coupled with tandem mass spectrometry experiments, and Jana Lee (Proteome Software) for assistance in using the Scaffold software.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Kimes, N., Grim, C., Johnson, W. et al. Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. ISME J 6, 835–846 (2012). https://doi.org/10.1038/ismej.2011.154
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2011.154
Keywords
This article is cited by
-
The road forward to incorporate seawater microbes in predictive reef monitoring
Environmental Microbiome (2024)
-
Holobiont responses of mesophotic precious red coral Corallium rubrum to thermal anomalies
Environmental Microbiome (2023)
-
Global Distribution of Hard Coral Pathogen Vibrio coralliilyticus; an Ensemble Modelling Approach
Thalassas: An International Journal of Marine Sciences (2023)
-
Over half of known human pathogenic diseases can be aggravated by climate change
Nature Climate Change (2022)
-
Integration of DSF and Temperature Signals for RpfC/RpfG Two-Component System Modulating Protease Production in Stenotrophomonas maltophilia FF11
Current Microbiology (2022)