Abstract
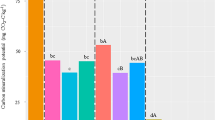
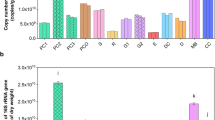
Priming effect (PE) is defined as a stimulation of the mineralization of soil organic matter (SOM) following a supply of fresh organic matter. This process can have important consequences on the fate of SOM and on the management of residues in agricultural soils, especially in tropical regions where soil fertility is essentially based on the management of organic matter. Earthworms are ecosystem engineers known to affect the dynamics of SOM. Endogeic earthworms ingest large amounts of soil and assimilate a part of organic matter it contains. During gut transit, microorganisms are transported to new substrates and their activity is stimulated by (i) the production of readily assimilable organic matter (mucus) and (ii) the possible presence of fresh organic residues in the ingested soil. The objective of our study was to see (i) whether earthworms impact the PE intensity when a fresh residue is added to a tropical soil and (ii) whether this impact is linked to a stimulation/inhibition of bacterial taxa, and which taxa are affected. A tropical soil from Madagascar was incubated in the laboratory, with a 13C wheat straw residue, in the presence or absence of a peregrine endogeic tropical earthworm, Pontoscolex corethrurus. Emissions of 12CO2 and 13CO2 were followed during 16 days. The coupling between DNA-SIP (stable isotope probing) and pyrosequencing showed that stimulation of both the mineralization of wheat residues and the PE can be linked to the stimulation of several groups especially belonging to the Bacteroidetes phylum.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Armas C, Ordiales R, Pugnaire FI . (2004). Measuring plant interactions: a new comparative index. Ecology 85: 2682–2686.
Barois I, Lavelle P . (1986). Changes in respiration rate and some physicochemical properties of a tropical soil during transit through Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta). Soil Biol Biochem 18: 539–541.
Beare MH, Reddy MV, Tian G, Srivastava SC . (1997). Agricultural intensification, soil biodiversity and agroecosystem function in the tropics: the role of decomposer biota. Appl Soil Ecol 6: 87–108.
Bernard L, Mougel C, Maron PA, Nowak V, Leveque J, Henault C et al. (2007). Dynamics and identification of soil microbial populations actively assimilating carbon from C-13-labelled wheat residue as estimated by DNA- and RNA-SIP techniques. Environ Microbiol 9: 752–764.
Bernard L, Maron PA, Mougel C, Nowak V, Leveque J, Marol C et al. (2009). Contamination of soil by copper affects the dynamics, diversity, and activity of soil bacterial communities involved in wheat decomposition and carbon storage. Appl Environ Microbiol 75: 7565–7569.
Blagodatskaya E, Kuzyakov Y . (2008). Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fert Soils 45: 115–131.
Blanchart E, Lavelle P, Braudeau E, Le Bissonnais Y, Valentin C . (1997). Regulation of soil structure by geophagous earthworm activities in humid savannas of Côte d’Ivoire. Soil Biol Biochem 29: 431–439.
Bodour AA, Wang JM, Brusseau ML, Maier RM . (2003). Temporal change in culturable phenanthrene degraders in response to long-term exposure to phenanthrene in a soil column system. Environ Microbiol 5: 888–895.
Bonkowski M, Griffiths BS, Ritz K . (2000). Food preferences of earthworms for soil fungi. Pedobiologia 44: 666–676.
Bossuyt H, Six J, Hendrix PF . (2004). Rapid incorporation of carbon from fresh residues into newly formed stable microaggregates within earthworm casts. Eur J Soil Sci 55: 393–399.
Bossuyt H, Six J, Hendrix PF . (2006). Interactive effects of functionally different earthworm species on aggregation and incorporation and decomposition of newly added residue carbon. Geoderma 130: 14–25.
Brookes PC, Cayuela ML, Contin M, De Nobili M, Kemmitt SJ, Mondini C . (2008). The mineralisation of fresh and humified soil organic matter by the soil microbial biomass. Waste Manage 28: 716–722.
Brown GG . (1995). How do earthworms affect microfloral and faunal community diversity? Plant Soil 170: 209–231.
Brown GG, Barois I, Lavelle P . (2000). Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactions with other edaphic functional domains. Eur J Soil Biol 36: 177–198.
Butenschoen O, Poll C, Langel R, Kandeler E, Marhan S, Scheu S . (2007). Endogeic earthworms alter carbon translocation by fungi at the soil-litter interface. Soil Biol Biochem 39: 2854–2864.
Chapuis-Lardy L, Brauman A, Bernard L, Pablo A, Toucet J, Mano M et al. (2010). Effect of the endogeic earthworm Pontoscolex corethrurus on the microbial structure and activity related to CO2 and N2O fluxes from a tropical soil (Madagascar). Appl Soil Ecol 45: 201–208.
Chapuis-Lardy L, Brossard M, Lavelle P, Schouller E . (1998). Phosphorus transformations in a Ferralsol through ingestion by Pontoscolex corethrurus, a geophagous earthworm. Eur J Soil Biol 34: 61–67.
Chapuis-Lardy L, Ramiandrisoa RS, Randriamanantsoa L, Morel C, Rabeharisoa L, Blanchart E . (2009). Modification of P availability by endogeic earthworms (Glossoscolecidae) in Ferralsols of the Malagasy Highlands. Biol Fert Soils 45: 415–422.
Coq S, Barthès BG, Oliver R, Rabary B, Blanchart E . (2007). Earthworm activity affects soil aggregation and soil organic matter dynamics according to the quality and localization of crop residues—an experimental study (Madagascar). Soil Biol Biochem 39: 2119–2128.
Egert M, Marhan S, Wagner B, Scheu S, Friedrich MW . (2004). Molecular profiling of 16S rRNA genes reveals diet-related differences of microbial communities in soil, gut, and casts of Lumbricus terrestris L (Oligochaeta: Lumbricidae). FEMS Microbiol Ecol 48: 187–197.
FAO . (1998). World Reference Base for Soil Resources. FAO, ISRIC, ISSS: Rome, pp 91.
Fontaine S, Mariotti A, Abbadie L . (2003). The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35: 837–843.
Guenet B, Leloup J, Raynaud X, Bardoux G, Abbadie L . (2010). Negative priming effect on mineralization in a soil free of vegetation for 80 years. Eur J Soil Sci 61: 384–391.
Jenkinson DS . (1966). Studies on decomposition of plant material in soil 2. Partial sterilization of soil and soil biomass. J Soil Sci 17: 280–302.
Jones CG, Lawton JH, Shachak M . (1994). Organisms as ecosystem engineers. Oikos 69: 373–386.
Kuzyakov Y . (2010). Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42: 1363–1371.
Kuzyakov Y, Friedel JK, Stahr K . (2000). Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32: 1485–1498.
Lauber CL, Hamady M, Knight R, Fierer N . (2009). Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microb 75: 5111–5120.
Lavelle P, Lattaud C, Trigo D, Barois I . (1994). Mutualism and biodiversity in soil. Plant Soil 170: 23–33.
Lavelle P . (1997). Faunal activities and soil processes: adaptive strategies that determine ecosystem function. Adv Ecol Res 27: 93–132.
Legendre P, Legendre L . (1998). Numerical Ecology, 2nd English edn Elsevier: Amsterdam.
Löhnis F . (1926). Nitrogen availability of green manures. Soil Sci 22: 253–290.
Moody SA, Briones MJI, Piearce TG, Dighton J . (1995). Selective consumption of decomposing wheat-straw by earthworms. Soil Biol Biochem 27: 1209–1213.
Moorhead DL, Sinsabaugh RL . (2006). A theoretical model of litter decay and microbial interaction. Ecol Monogr 76: 151–174.
Pankratov TA, Tindall BJ, Liesack W, Dedysh SN . (2007). Mucilaginibacter paludis gen. nov., sp. nov. and Mucilaginibacter gracilis sp. nov., pectin-, xylan- and laminarin-degrading members of the family Sphingobacteriaceae from acidic Sphagnum peat bog. Int J Syst Evol Micr 57: 2979–2979.
Panikov NS . (2010). Microbial ecology. In: Wang LK et al. (eds) Handbook of Environmental Engineering, Volume 10: Environmental Biotechnology. Springer Science+Business Media, LLC: New York, NY, doi: 10.10007/978-1-60327-140-0_4.
Patron JC, Sanchez P, Brown GC, Brossard M, Barois I, Gutierrez C . (1999). Phosphorus in soil and Brachiaria decumbens plants as affected by the geophagous earthworm Pontoscolex corethrurus and P fertilization. Pedobiologia 43: 547–556.
Roesch LF, Fulthorpe RR, Riva A, Casella G, Hadwin AKM, Kent AD et al. (2007). Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J 1: 283–290.
Speratti AB, Whalen JK . (2008). Carbon dioxide and nitrous oxide fluxes from soil as influenced by anecic and endogeic earthworms. Appl Soil Ecol 38: 27–33.
Weon HY, Kim BY, Yoo SH, Kwon SW, Go SJ, Stackebrandt E . (2008). Uliginosibacterium gangwonense gen. nov., sp nov., isolated from a wetland, Yongneup, in Korea. Int J Syst Evol Micr 58: 131–135.
Wilson DB . (2009). Evidence for a novel mechanism of microbial cellulose degradation. Cellulose 16: 723–727.
Xu L, Teng Y, Li ZG, Norton JM, Luo YM . (2010). Enhanced removal of polychlorinated biphenyls from alfalfa rhizosphere soil in a field study: the impact of a rhizobial inoculum. Sci Total Environ 408: 1007–1013.
Yang HC, Im WT, An DS, Park WS, Kim IS, Lee ST . (2005). Silvimonas terrae gen. nov., sp nov., a novel chitindegrading facultative anaerobe belonging to the ‘Betaproteobacteria’. Int J Syst Evol Micr 55: 2329–2332.
Acknowledgements
This work has been partly funded by the French ‘Agence Nationale pour la Recherche’ (program Génomique) and the ‘Institut National des Sciences de l’Univers’ (Appel d’Offre EC2CO, program MicroBien). We thank K Lemoal and the LRI staff in Madagascar for their technical help for the experiment settlement. We also thank P Tillard (BPMP, INRA, Montpellier, Fr) for the IRMS analyses and V Santoni (BPMP, INRA, Montpellier, Fr) to give us the access to the ultracentrifuge and helping to use it.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Bernard, L., Chapuis-Lardy, L., Razafimbelo, T. et al. Endogeic earthworms shape bacterial functional communities and affect organic matter mineralization in a tropical soil. ISME J 6, 213–222 (2012). https://doi.org/10.1038/ismej.2011.87
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2011.87
Keywords
This article is cited by
-
Valorization of Cow Manure: Unraveling Bacterial Community Changes Driven by Vermicomposting and Their Impact on Vermicompost Tea Production
Waste and Biomass Valorization (2023)
-
Soil environment reshapes microbiota of laboratory-maintained Collembola during host development
Environmental Microbiome (2022)
-
Changes in soil carbon mineralization related to earthworm activity depend on the time since inoculation and their density in soil
Scientific Reports (2022)
-
Diversity and predicted functional roles of cultivable bacteria in vermicompost: bioprospecting for potential inoculum
Archives of Microbiology (2022)
-
Effects of Phyllostachys pubescens expansion on underground soil fauna community and soil food web in a Cryptomeria japonica plantation, Lushan Mountain, subtropical China
Journal of Soils and Sediments (2021)