Abstract
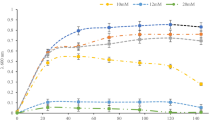
The copper membrane monooxygenases (CuMMOs) are an important group of enzymes in environmental science and biotechnology. Areas of relevance include the development of green chemistry for sustainable exploitation of methane (CH4) reserves, remediation of chlorinated hydrocarbon contamination and monitoring human impact in the biogeochemical cycles of CH4 and nitrogen. Challenges for all these applications are that many aspects of the ecology, physiology and structure–function relationships in the CuMMOs are inadequately understood. Here, we describe genetic and physiological characterization of a novel member of the CuMMO family that has an unusual physiological substrate range (C2–C4 alkanes) and a distinctive bacterial host (Mycobacterium). The Mycobacterial CuMMO genes (designated hmoCAB) were amenable to heterologous expression in M. smegmatis—this is the first example of recombinant expression of a complete and highly active CuMMO enzyme. The apparent specific activity of recombinant cells containing hmoCAB ranged from 2 to 3 nmol min–1 per mg protein on ethane, propane and butane as substrates, and the recombinants could also attack ethene, cis-dichloroethene and 1,2-dichloroethane. No detectable activity of recombinants or wild-type strains was seen with methane. The specific inhibitor allylthiourea strongly inhibited growth of wild-type cells on C2–C4 alkanes, and omission of copper from the medium had a similar effect, confirming the physiological role of the CuMMO for growth on alkanes. The hydrocarbon monooxygenase provides a new model for studying this important enzyme family, and the recombinant expression system will enable biochemical and molecular biological experiments (for example, site-directed mutagenesis) that were previously not possible.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Avrahami S, Bohannan BJM . (2009). N2O emission rates in a California meadow soil are influenced by fertilizer level, soil moisture and the community structure of ammonia-oxidizing bacteria. Glob Change Biol 15: 643–655.
Baani M, Liesack W . (2008). Two isozymes of particulate methane monooxygenase with different methane oxidation kinetics are found in Methylocystis sp strain SCZ. Proc Natl Acad Sci USA 105: 10203–10208.
Balasubramanian R, Smith SM, Rawat S, Yatsunyk LA, Stemmler TL, Rosenzweig AC . (2010). Oxidation of methane by a biological dicopper centre. Nature 465: 115–121.
Bedard C, Knowles R . (1989). Physiology, biochemistry, and specific inhibitors of Ch4, Nh4+, and co-oxidation by methanotrophs and nitrifiers. Microbiol Revs 53: 68–84.
Coleman NV, Mattes TE, Gossett JM, Spain JC . (2002a). Phylogenetic and kinetic diversity of aerobic vinyl chloride-assimilating bacteria from contaminated sites. Appl Environ Microbiol 68: 6162–6171.
Coleman NV, Mattes TE, Gossett JM, Spain JC . (2002b). Biodegradation of cis-dichloroethene as the sole carbon source by a beta-proteobacterium. Appl Environ Microbiol 68: 2726–2730.
Coleman NV, Bui NB, Holmes AJ . (2006). Soluble di-iron monooxygenase gene diversity in soils, sediments and ethene enrichments. Environ Microbiol 8: 1228–1239.
Coleman NV, Yau S, Wilson NL, Nolan LM, Migocki MD, Ly M et al. (2011). Untangling the multiple monooxygenases of Mycobacterium chubuense strain NBB4, a versatile hydrocarbon degrader. Environ Microbiol Reports 3: 297–307.
Conrad R . (1996). Soil microorganisms as controllers of atmospheric trace gases (H-2, CO, CH4, OCS, N2O, and NO). Microbiol Revs 60: 609–640.
Dumont MG, Radajewski SM, Miguez CB, McDonald IR, Murrell JC . (2006). Identification of a complete methane monooxygenase operon from soil by combining stable isotope probing and metagenomic analysis. Environ Microbiol 8: 1240–1250.
Dunfield PF, Yuryev A, Senin P, Smirnova AV, Stott MB, Hou SB et al. (2007). Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 450: 879–882.
Ettwig KF, Butler MK, Le Paslier D, Pelletier D, Mangenot S, Kuypers MMM et al. (2010). Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464: 543–548.
Furuya T, Hirose S, Osanai H, Semba H, Kino K . (2011). Identification of the monooxygenase gene clusters responsible for the regioselective oxidation of phenol to hydroquinone in mycobacteria. Appl Environ Microbiol 77: 1214–1220.
Gilch S, Vogel M, Lorenz MW, Meyer O, Schmidt I . (2009). Interaction of the mechanism-based inactivator acetylene with ammonia monooxygenase of Nitrosomonas europaea. Microbiology 155: 279–284.
Gou ZX, Xing XH, Luo MF, Jiang H, Han B, Wu H et al. (2006). Functional expression of the particulate methane mono-oxygenase gene in recombinant Rhodococcus erythropolis. FEMS Microbiol Letts 263: 136–141.
Guengerich FP, Crawford WM, Watanabe PG . (1979). Activation of vinyl-chloride to covalently bound metabolites—roles of 2-chloroethylene oxide and 2-chloroacetaldehyde. Biochemistry 18: 5177–5182.
Hakemian AS, Rosenzweig AC . (2007). The biochemistry of methane oxidation. Ann Rev Biochem 76: 223–241.
Hakemian AS, Kondapalli KC, Telser J, Hoffman BM, Stemmler TL, Rosenzweig AC . (2008). The metal clusters of particulate methane monooxygenase from Methylosinus trichosporium OB3b. Biochem 47: 6793–6801.
Hamamura N, Page C, Long T, Semprini L, Arp DJ . (1997). Chloroform cometabolism by butane-grown CF8, Pseudomonas butanovora, and Mycobacterium vaccae JOB5 and methane-grown Methylosinus trichosporium OB3b. Appl Environ Microbiol 63: 3607–3613.
Hamamura N, Storfa RT, Semprini L, Arp D . (1999). Diversity in butane monooxygenases among butane-grown bacteria. Appl Environ Microbiol 65: 4586–4593.
Hamamura N, Yeager CM, Arp DJ . (2001). Two distinct monooxygenases for alkane oxidation in Nocardioides sp strain CF8. Appl Environ Microbiol 67: 4992–4998.
Holmes AJ, Costello A, Lidstrom ME, Murrell JC . (1995). Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Letts 132: 203–208.
Holmes AJ, Roslev P, McDonald IR, Iversen N, Henriksen K, Murrell JC . (1999). Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl Environ Microbiol 65: 3312–3318.
Kalb VF, Bernlohr RW . (1977). A new spectrophotometric assay for protein in cell extracts. Anal Biochem 82: 362–371.
Kip N, van Winden JF, Pan Y, Bodrossy L, Reichart GJ, Smolders AJP et al. (2010). Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nat Geoscience 3: 617–621.
Konneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA . (2005). Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437: 543–546.
Lapierre P, Gogarten JP . (2009). Estimating the size of the bacterial pan-genome. Trends Genet 25: 107–110.
Le N, Coleman NV . (2011). Biodegradation of vinyl chloride, cis-dichloroethene and 1,2-dichloroethane in the alkene/alkane-oxidising Mycobacterium strain NBB4. Biodegradation. doi:10.1007/s10532-1011-9466-0.
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW et al. (2006). Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442: 806–809.
Lieberman RL, Rosenzweig AC . (2005). Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 434: 177–182.
Luke C, Krause S, Cavigiolo S et al. (2010). Biogeography of wetland rice methanotrophs. Environ Microbiol 12: 862–872.
Ly MA, Coleman NV . (2011). Construction and evaluation of pMycoFos, a fosmid shuttle vector for Mycobacterium spp. with inducible gene expression and copy number control. J Microbiol Meth. doi:10.1016/j.mimet.2011.06.005.
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA . (2009). Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461: 976–979.
Mattes TE, Alexander AK, Coleman NV . (2010). Aerobic biodegradation of the chloroethenes: pathways, enzymes, ecology, and evolution. FEMS Microbiol Revs 34: 445–475.
McCarty GW . (1999). Modes of action of nitrification inhibitors. Biol Fert Soils 29: 1–9.
Menyailo OV, Hungate BA, Abraham WR, Conrad R . (2008). Changing land use reduces soil CH4 uptake by altering biomass and activity but not composition of high-affinity methanotrophs. Glob Change Biol 14: 2405–2419.
Morrill TC, Friedrich LE, Machonkin MA, Whitbourne JE, Eastman CA . (1981). N-(2-hydroxyethyl)-4-(p-nitrobenzylidene)-1,4-dihydropyridine (DHP) from the reaction of 4-(p-nitrobenzyl)pyridine (NBP) with ethylene oxide. J Heterocyclic Chem 18: 1645–1647.
Nielsen AK, Gerdes K, Murrell JC . (1997). Copper-dependent reciprocal transcriptional regulation of methane monooxygenase genes in Methylococcus capsulatus and Methylosinus trichosporium. Mol Microbiol 25: 399–409.
Pol A, Heijmans K, Harhangi HR, Tedesco D, Jetten MSM, Op den Camp HJM . (2007). Methanotrophy below pH1 by a new Verrucomicrobia species. Nature 450: 874–878.
Rotthauwe JH, Witzel KP, Liesack W . (1997). The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63: 4704–4712.
Santoro AE, Casciotti KL, Francis CA . (2010). Activity, abundance and diversity of nitrifying archaea and bacteria in the central California Current. Environ Microbiol 12: 1989–2006.
Sayavedra-Soto LA, Hamamura N, Liu C-W, Kimbrel JA, Chang JH, Arp DJ . (2011). The membrane-associated monooxygenase in the butane-oxidizing Gram-positive bacterium Nocardioides sp. strain CF8 is a novel member of the AMO/PMO family. Environ Microbiol Reports. doi:10.1111/j.1758-2229.2010.00239.x.
Semrau JD, Chistoserdov A, Lebron J, Costello A, Davagnino J, Kenna E et al. (1995). Particulate methane monooxygenase genes in methanotrophs. J Bacteriol 177: 3071–3079.
Semrau JD, DiSpirito AA, Yoon S . (2010). Methanotrophs and copper. FEMS Microbiol Revs 34: 496–531.
Singh BK, Bardgett RD, Smith P, Reay DS . (2010). Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Revs Microbiol 8: 779–790.
Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs Jr WR . (1990). Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol 4: 1911–1919.
Squillace PJ, Moran MJ, Lapham WW, Price CV, Clawges RM, Zogorski JS . (1999). Volatile organic compounds in untreated ambient groundwater of the United States, 1985–1995. Environ Sci Technol 33: 4176–4187.
Stoecker K, Bendinger B, Schoning B, Nielsen PH, Nielsen JL, Baranyi C et al. (2006). Cohn′s Crenothrix is a filamentous methane oxidizer with an unusual methane monooxygenase. Proc Natl Acad Sci USA 103: 2363–2367.
Tavormina PL, Ussler W, Joye SB, Harrison BK, Orphan VJ . (2010). Distributions of putative aerobic methanotrophs in diverse pelagic marine environments. ISME J 4: 700–710.
Triccas JA, Parish T, Britton WJ, Gicquel B . (1998). An inducible expression system permitting the efficient purification of a recombinant antigen from Mycobacterium smegmatis. FEMS Microbiol Lett 167: 151–156.
Wild J, Hradecna Z, Szybalski W . (2002). Conditionally amplifiable BACs: switching from single-copy to high-copy vectors and genomic clones. Genome Res 12: 1434–1444.
Zahn JA, DiSpirito AA . (1996). Membrane-associated methane monooxygenase from Methylococcus capsulatus (Bath). J Bacteriol 178: 1018–1029.
Zhang LM, Offre PR, He JZ, Verhamme DT, Nicol GW, Prosser JI . (2010). Autotrophic ammonia oxidation by soil Thaumarchaea. Proc Natl Acad Sci USA 107: 17240–17245.
Acknowledgements
This work was supported by grants from the Australian Research Council to NVC and AJH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Coleman, N., Le, N., Ly, M. et al. Hydrocarbon monooxygenase in Mycobacterium: recombinant expression of a member of the ammonia monooxygenase superfamily. ISME J 6, 171–182 (2012). https://doi.org/10.1038/ismej.2011.98
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2011.98
Keywords
This article is cited by
-
Verrucomicrobial methanotrophs grow on diverse C3 compounds and use a homolog of particulate methane monooxygenase to oxidize acetone
The ISME Journal (2021)
-
Research progress in bioremediation of petroleum pollution
Environmental Science and Pollution Research (2021)
-
Novel copper-containing membrane monooxygenases (CuMMOs) encoded by alkane-utilizing Betaproteobacteria
The ISME Journal (2020)
-
Characterization of a long overlooked copper protein from methane- and ammonia-oxidizing bacteria
Nature Communications (2018)
-
Survey of methanotrophic diversity in various ecosystems by degenerate methane monooxygenase gene primers
AMB Express (2017)