Abstract
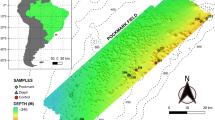
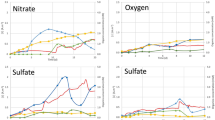
Sediment-hosting hydrothermal systems in the Okinawa Trough maintain a large amount of liquid, supercritical and hydrate phases of CO2 in the seabed. The emission of CO2 may critically impact the geochemical, geophysical and ecological characteristics of the deep-sea sedimentary environment. So far it remains unclear whether microbial communities that have been detected in such high-CO2 and low-pH habitats are metabolically active, and if so, what the biogeochemical and ecological consequences for the environment are. In this study, RNA-based molecular approaches and radioactive tracer-based respiration rate assays were combined to study the density, diversity and metabolic activity of microbial communities in CO2-seep sediment at the Yonaguni Knoll IV hydrothermal field of the southern Okinawa Trough. In general, the number of microbes decreased sharply with increasing sediment depth and CO2 concentration. Phylogenetic analyses of community structure using reverse-transcribed 16S ribosomal RNA showed that the active microbial community became less diverse with increasing sediment depth and CO2 concentration, indicating that microbial activity and community structure are sensitive to CO2 venting. Analyses of RNA-based pyrosequences and catalyzed reporter deposition-fluorescence in situ hybridization data revealed that members of the SEEP-SRB2 group within the Deltaproteobacteria and anaerobic methanotrophic archaea (ANME-2a and -2c) were confined to the top seafloor, and active archaea were not detected in deeper sediments (13–30 cm in depth) characterized by high CO2. Measurement of the potential sulfate reduction rate at pH conditions of 3–9 with and without methane in the headspace indicated that acidophilic sulfate reduction possibly occurs in the presence of methane, even at very low pH of 3. These results suggest that some members of the anaerobic methanotrophs and sulfate reducers can adapt to the CO2-seep sedimentary environment; however, CO2 and pH in the deep-sea sediment were found to severely impact the activity and structure of the microbial community.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA . (1990). Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56: 1919–1925.
Amann RI, Ludwig W, Schleifer KH . (1995). Phylogenetic identification and in-situ detection of individual microbial-cells without cultivation. Microbiol Rev 59: 143–169.
Barry JP, Buck KR, Lovera CF, Kuhnz L, Whaling PJ, Peltzer ET et al. (2004). Effects of direct ocean CO2 injection on deep-sea meiofauna. J Oceanogr 60: 759–766.
Biddle JF, Cardman Z, Mendlovitz H, Albert DB, Lloyd KG, Boetius A et al. (2012). Anaerobic oxidation of methane at different temperature regimes in Guaymas Basin hydrothermal sediments. ISME J 6: 1018–1031.
Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A et al. (2000). A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407: 623–626.
Brazelton WJ, Schrenk MO, Kelley DS, Baross JA . (2006). Methane- and sulfur-metabolizing microbial communities dominate the Lost City hydrothermal field ecosystem. Appl Environ Microbiol 72: 6257–6270.
Chao A . (1987). Estimating the population-size for capture-recapture data with unequal catchability. Biometrics 43: 783–791.
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ et al. (2009). The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37: D141–D145.
de Beer D, Sauter E, Niemann H, Kaul N, Foucher J, Witte U et al. (2006). In situ fluxes and zonation of microbial activity in surface sediments of the Håkon Mosby Mud Volcano. Limnol Oceanogr 51: 1315–1331.
de Beer D, Schramm A, Santegoeds CM, Kühl M . (1997). A nitrite microsensor for profiling environmental biofilms. Appl Environ Microbiol 63: 973–977.
DeLong EF . (1992). Archaea in coastal marine environments. Proc Natl Acad Sci USA 89: 5685–5689.
Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G et al. (2011). Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Chang 1: 165–169.
Fossing H, Jørgensen BB . (1989). Measurement of bacterial sulfate reduction in sediments—evaluation of a single-step chromium reduction method. Biogeochemistry 8: 205–222.
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ . (2008). Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol 74: 2461–2470.
Grasshoff K, Ehrhardt M, Kremling K . (1999) Methods of Seawater Analysis. Wiley-VCH: Weinheim, pp 600.
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM et al. (2008). Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454: 96–99.
Holler T, Widdel F, Knittel K, Amann R, Kellermann MY, Hinrichs K-U et al. (2011). Thermophilic anaerobic oxidation of methane by marine microbial consortia. ISME J 5: 1946–1956.
Hoshino T, Morono Y, Terada T, Imachi H, Ferdelman TG, Inagaki F . (2011). Comparative study of subseafloor microbial community structures in deeply buried coral fossils and sediment matrices from the Challenger Mound in the Porcupine Seabight. Front Microbiol 2: 231.
House KZ, Schrag DP, Harvey CF, Lackner KS . (2006). Permanent carbon dioxide storage in deep-sea sediments. Proc Natl Acad Sci USA 103: 12291–12295.
Huber T, Faulkner G, Hugenholtz P . (2004). Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20: 2317–2319.
Inagaki F, Kuypers MMM, Tsunogai U, Ishibashi J, Nakamura K, Treude T et al. (2006). Microbial community in a sediment-hosted CO2 lake of the southern Okinawa Trough hydrothermal system. Proc Natl Acad Sci USA 103: 14164–14169.
Inagaki F, Sakihama Y, Inoue A, Kato C, Horikoshi K . (2002). Molecular phylogenetic analysis of reverse-transcribed bacterial rRNA obtained from deep-sea cold seep sediments. Environ Microbiol 4: 277–286.
Inagaki F, Takai K, Nealson KH, Horikoshi K . (2003). Sulfurimonas autotrophica gen. nov., sp. nov., a sulfur and thiosulfate oxidizing epsilon proteobacterium isolated from the Okinawa Trough. Int J Syst Evol Microbiol 53: 1801–1805.
Jeroschewski P, Steuckart C, Kühl M . (1996). An amperometric microsensor for the determination of H2S in aquatic environments. Anal Chem 68: 4351–4357.
Kallmeyer J, Boetius A . (2004). Effects of temperature and pressure on sulfate reduction and anaerobic oxidation of methane in hydrothermal sediments of Guaymas Basin. Appl Environ Microbiol 70: 1231–1233.
Kallmeyer J, Ferdelman TG, Weber A, Fossing H, Jørgensen BB . (2004). A cold chromium distillation procedure for radiolabeled sulfide applied to sulfate reduction measurements. Limnol Oceanogr: Methods 2: 171–180.
Kallmeyer J, Smith DC, Spivack AJ, D’Hondt S . (2008). New cell extraction procedure applied to deep subsurface sediments. Limnol Oceanogr: Methods 6: 236–245.
Kleindienst S, Ramette A, Amann R, Knittel K . (2012). Distribution and in situ abundance of sulfate-reducing bacteria in diverse marine hydrocarbon seep sediments. Environ Microbiol 14: 2689–2710.
Knittel K, Boetius A . (2009). Anaerobic oxidation of methane: progress with an unknown process. Annu Rev Microbiol 63: 311–334.
Knittel K, Boetius A, Lemke A, Eilers H, Lochte K, Pfannkuche O et al. (2003). Activity, distribution, and diversity of sulfate reducers and other bacteria in sediments above gas hydrate (Cascadia margin, Oregon). Geomicrobiol J 20: 269–294.
Konno U, Tsunogai U, Nakagawa F, Nakaseama M, Ishibashi J, Nunoura T et al. (2006). Liquid CO2 venting on the seafloor: Yonaguni knoll IV hydrothermal system, Okinawa Trough. Geophys Res Lett 33: L16607.
Krebs CJ . (1989) Ecological Methodology 2nd edn Benjamin/Cummings: Menlo Park, CA, USA.
Lloyd KG, Albert DB, Biddle JF, Chanton JP, Pizarro O, Teske A . (2010). Spatial structure and activity of sedimentary microbial communities underlying a Beggiatoa spp. mat in a Gulf of Mexico hydrocarbon seep. PLoS ONE 5: e8738.
Lloyd KG, Lapham L, Teske A . (2006). Anaerobic methane-oxidizing community of ANME-1b archaea in hypersaline Gulf of Mexico sediments. Appl Environ Microbiol 72: 7218–7230.
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar et al. (2004). ARB: a software environment for sequence data. Nucleic Acids Res 32: 1363–1371.
Lupton J, Butterfield D, Lilley M, Evans L, Nakamura K, Chadwick W et al. (2006). Submarine venting of liquid carbon dioxide on a Mariana Arc volcano. Geochem Geophy Geosy 7: Q08007.
Lupton J, Lilley M, Butterfield D, Evans L, Embley R, Massoth G et al. (2008). Venting of a separate CO2-rich gas phase from submarine arc volcanoes: examples from the Mariana and Tonga-Kermadec arcs. J Geophys Res 113: B8S12.
Manz W, Eisenbrecher M, Neu TR, Szewzyk U . (1998). Abundance and spatial organization of Gram-negative sulfate-reducing bacteria in activated sludge investigated by in situ probing with specific 16S rRNA targeted oligonucleotides. FEMS Microbiol Ecol 25: 43–61.
Miyashita A, Mochimaru H, Kazama H, Ohashi A, Yamaguchi T, Nunoura T et al. (2009). Development of 16S rRNA gene-targeted primers for detection of archaeal anaerobic methanotrophs (ANMEs). FEMS Microbiol Lett 297: 31–37.
Morono Y, Inagaki F . (2010). Automatic slide-loader fluorescence microscope for discriminative enumeration of subseafloor life. Sci Drilling 9: 32–36.
Morono Y, Terada T, Masui N, Inagaki F . (2009). Discriminative detection and enumeration of microbial life in marine subsurface sediments. ISME J 3: 503–511.
Nauhaus K, Treude T, Boetius A, Krüger M . (2005). Environmental regulation of the anaerobic oxidation of methane: a comparison of ANME-I and ANME-II communities. Environ Microbiol 7: 98–106.
Nealson KH . (2006). Lakes of liquid CO2 in the deep sea. Proc Natl Acad Sci USA 103: 13903–13904.
Niemann H, Lösekann T, de Beer D, Elvert M, Nadalig T, Knittel K et al. (2006). Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature 443: 854–858.
Nunoura T, Oida H, Nakaseama M, Kosaka A, Ohkubo SB, Kikuchi T et al. (2010). Archaeal diversity and distribution along thermal and geochemical gradients in hydrothermal sediments at the Yonaguni Knoll IV hydrothermal field in the southern Okinawa Trough. Appl Environ Microbiol 76: 1198–1211.
Nunoura T, Takai K . (2009). Comparison of microbial communities associated with phase-separation-induced hydrothermal fluids at the Yonaguni Knoll IV hydrothermal field, the Southern Okinawa Trough. FEMS Microbiol Ecol 67: 351–370.
Nunoura T, Takaki Y, Kazama H, Hirai M, Ashi J, Imachi H et al. (2012). Microbial diversity in deep-sea methane seep sediments presented by SSU rRNA gene tag sequencing. Microb Environ doi:10.1264/jsme2.ME12032.
Onstott TC . (2005). Impact of CO2 injections on deep subsurface microbial ecosystems and potential ramifications for the subsurface biosphere. In: Thomas DC, Benson SM, (eds) Carbon Dioxide Capture for Storage in Deep Geologic Formation-Results from the CO2 Capture Project: Vol. 2, Geologic Storage of Carbon Dioxide with Monitoring and Verification. Elsevier: London, pp 1207–1239.
Orphan VJ, House CH, Hinrichs KU, McKeegan KD, DeLong EF . (2002). Multiple archaeal groups mediate methane oxidation in anoxic cold seep sediments. Proc Natl Acad Sci USA 99: 7663–7668.
Pernthaler A, Pernthaler J, Amann R . (2002). Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol 68: 3094–3101.
Pielou EC . (1966). Species-diversity and pattern-diversity in study of ecological succession. J Theor Biol 10: 370–383.
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig WG, Peplies J et al. (2007). SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35: 7188–7196.
Rehder G, Schneider von Deimling J, Cruise participants (2008), RV Sonne Cruise Report SO 196, SUMSUN 2008, Suva Guam Okinawa Trough Manila, 19 February —26 March, 2008, Leibniz Institut für Ostseeforschung: Sektion Meereschemie, Warnemünde.
Revsbech NP, Ward DM . (1983). Oxygen microelectrode that is insensitive to medium chemical composition: use in an acid microbial mat dominated by Cyanidium caldarium. Appl Environ Microbiol 45: 755–759.
Sakai H, Gamo T, Kim ES, Tsutsumi M, Tanaka T, Ishibashi J et al. (1990). Venting of carbon-dioxide rich fluid and hydrate formation in mid-Okinawa Trough backarc basin. Science 248: 1093–1096.
Schenke, Werner H, Rehder G . (2008). Swath sonar bathymetry during Sonne cruise SO196 with links to multibeam raw data files. Alfred Wegener Institute for Polar and Marine Research: Bremerhaven doi:10.1594/PANGAEA.701955.
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: 7537–7541.
Shitashima K, Maeda Y, Koike Y, Ohsumi T . (2008). Natural analogue of the rise and dissolution of liquid CO2 in the ocean. Int J Greenhouse Gas Control 2: 95–104.
Suzuki R, Ishibashi J, Nakaseama M, Konno U, Tsunogai U, Gena K et al. (2008). Diverse range of mineralization induced by phase separation of hydrothermal fluid: a case study of the Yonaguni Knoll IV hydrothermal field in the Okinawa Trough back-arc basin. Res Geol 58: 267–288.
Teske A, Hinrichs K-U, Edgcomb V, Gomez AD, Kysela D, Sylva SP et al. (2002). Microbial diversity of hydrothermal sediments in the Guaymas Basin: evidence for anaerobic methanotrophic communities. Appl Environ Microbiol 68: 1994–2007.
Tunnicliffe V, Davies KTA, Butterfield DA, Embley RW, Rose JM, Chadwick WW . (2009). Survival of mussels in extremely acidic waters on a submarine volcano. Nature Geosci 2: 344–348.
Yanagawa K, Sunamura M, Lever MA, Morono Y, Hiruta A, Ishizaki O et al. (2011). Niche separation of methanotrophic archaea (ANME-1 and -2) in methane-seep sediments of the eastern Japan Sea offshore Joetsu. Geomicrobiol J 28: 118–129.
Yu YN, Breitbart M, McNairnie P, Rohwer F . (2006). FastGroupII: a web-based bioinformatics platform for analyses of large 16S rDNA libraries. BMC Bioinformatics 7: 57.
Wenzhöfer F, Holby O, Glud RN, Nielsen HK, Gundersen JK . (2000). In situ microsensor studies of a shallow water hydrothermal vent at Milos, Greece. Mar Chem 69: 43–54.
Acknowledgements
We thank the shipboard science party of the SO196 cruise and the crew and operation teams of the RV Sonne and ROV Quest 4000 for their support in sample collection. We also thank Noriaki Masui for technical assistance. We are grateful to Katrin Knittel and Sara Kleindienst for helpful discussion regarding CARD-FISH using the SEEP2-658 probe. This work was supported in part by a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS) Fellows (20-10764, to KY), a Grant-in-Aid for Scientific Research: Project TAIGA (New Scientific Research on Innovative Areas, 20109003, to MS and TU), the JSPS Funding Program for the Next Generation of World-Leading Researchers (NEXT Program, to FI), the JSPS Strategic Fund for Strengthening Leading-edge Research and Development (to JAMSTEC), the Max Planck Society (MPG project funds) and project SUMSUN (grant no. 03G0196B), funded by the German Federal Ministry of Education and Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Yanagawa, K., Morono, Y., de Beer, D. et al. Metabolically active microbial communities in marine sediment under high-CO2 and low-pH extremes. ISME J 7, 555–567 (2013). https://doi.org/10.1038/ismej.2012.124
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2012.124
Keywords
This article is cited by
-
Microbial carbon-capture cells for wastewater treatment: a step towards environmental sustainability
Biomass Conversion and Biorefinery (2022)
-
Metal-Tolerant Fungal Communities Are Delineated by High Zinc, Lead, and Copper Concentrations in Metalliferous Gobi Desert Soils
Microbial Ecology (2020)
-
Structural and Functional Changes of Groundwater Bacterial Community During Temperature and pH Disturbances
Microbial Ecology (2019)
-
Structure design and performance comparison of large-scale marine sediment microbial fuel cells in lab and real sea as power source to drive monitoring instruments for long-term work
Ionics (2018)
-
Bacterial community profile of contaminated soils in a typical antimony mining site
Environmental Science and Pollution Research (2018)