Abstract
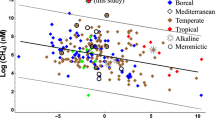
Soda lakes are saline and alkaline ecosystems that are believed to have existed throughout the geological record of Earth. They are widely distributed across the globe, but are highly abundant in terrestrial biomes such as deserts and steppes and in geologically interesting regions such as the East African Rift valley. The unusual geochemistry of these lakes supports the growth of an impressive array of microorganisms that are of ecological and economic importance. Haloalkaliphilic Bacteria and Archaea belonging to all major trophic groups have been described from many soda lakes, including lakes with exceptionally high levels of heavy metals. Lonar Lake is a soda lake that is centered at an unusual meteorite impact structure in the Deccan basalts in India and its key physicochemical and microbiological characteristics are highlighted in this article. The occurrence of diverse functional groups of microbes, such as methanogens, methanotrophs, phototrophs, denitrifiers, sulfur oxidizers, sulfate reducers and syntrophs in soda lakes, suggests that these habitats harbor complex microbial food webs that (a) interconnect various biological cycles via redox coupling and (b) impact on the production and consumption of greenhouse gases. Soda lake microorganisms harbor several biotechnologically relevant enzymes and biomolecules (for example, cellulases, amylases, ectoine) and there is the need to augment bioprospecting efforts in soda lake environments with new integrated approaches. Importantly, some saline and alkaline lake ecosystems around the world need to be protected from anthropogenic pressures that threaten their long-term existence.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Andersen GL, He Z, Desantis TZ, Brodie EL, Zhou J . (2010). The use of microarrays in microbial ecology. In: Liu WT, Jansson JK (eds). Environmental Molecular Microbiology. Caister Academic Press: Norwich,, pp. 87–110.
Antony CP, Kumaresan D, Ferrando L, Boden R, Moussard H, Scavino AF et al. (2010). Active methylotrophs in the sediments of Lonar Lake, a saline and alkaline ecosystem formed by meteor impact. ISME J 4: 1470–1480.
Antony CP, Doronina NV, Boden R, Trotsenko YA, Shouche YS, Murrell JC . (2012a). Methylophaga lonarensis, a novel moderately haloalkaliphilic methylotroph isolated from the soda lake sediments of a meteorite impact crater. Int J Syst Evol Microbiol 62: 1613–1618.
Antony CP, Murrell JC, Shouche YS . (2012b). Molecular diversity of methanogens and identification of Methanolobus sp. as active methylotrophic Archaea in Lonar crater lake sediments. FEMS Microbiol Ecol 81: 43–51.
Asao M, Pinkart HC, Madigan MT . (2011). Diversity of extremophilic purple phototrophic bacteria in Soap Lake, a Central Washington (USA) Soda Lake. Environ Microbiol 13: 2146–2157.
Cadillo-Quiroz H, Yashiro E, Yavitt JB, Zinder SH . (2008). Characterization of the archaeal community in a minerotrophic fen and terminal restriction fragment length polymorphism-directed isolation of a novel hydrogenotrophic methanogen. Appl Environ Microbiol 74: 2059–2068.
Carini SA, Joye SB . (2008). Nitrification in Mono Lake, California: Activity and community composition during contrasting hydrological regimes. Limnol Oceanogr 53: 2546–2557.
Chakravarty I . (2009). Conservation and management for the Lonar crater-lake, Maharashtra, India. S Asian J Tour Herit 2: 112–118.
Ciulla RA, Diaz MR, Taylor BF, Roberts MF . (1997). Organic osmolytes in aerobic bacteria from Mono Lake, an alkaline, moderately hypersaline environment. Appl Environ Microbiol 63: 220–226.
Couradeau E, Benzerara K, Gerard E, Moreira D, Bernard S, Brown GE, López-Garcia P . (2012). An early-branching microbialite cyanobacterium forms intracellular carbonates. Science 336: 459–462.
Dedysh SN, Pankratov TA, Belova SE, Kulichevskaya IS, Liesack W . (2006). Phylogenetic analysis and in situ identification of bacteria community composition in an acidic Sphagnum peat bog. Appl Environ Microbiol 72: 2110–2117.
Doronina NV, Darmaeva T, Trotsenko YA . (2001). New aerobic methylotrophic isolates from the Soda lakes of the southern Transbaikal. Mikrobiologiia 70: 398–404.
Doronina NV, Darmaeva TD, Trotsenko YA . (2003). Methylophaga alcalica sp. nov., a novel alkaliphilic and moderately halophilic, obligately methylotrophic bacterium from an East Mongolian saline soda lake. Int J Syst Evol Microbiol 53: 223–229.
Drake HL, Gößner AS, Daniel SL . (2008). Old acetogens, new light. In: Wiegel J, Maier RJ, Adams MWW (eds). Incredible Anaerobes: From Physiology to Genomics to Fuels. New York Academy of Sciences: Boston, MA, pp. 100–128.
Drake HL, Horn MA, Wüst PK . (2009). Intermediary ecosystem metabolism as a main driver of methanogenesis in acidic wetland soil. Environ Microbiol Rep 1: 307–318.
Duckworth AW, Grant WD, Jones BE, Van Steenbergen R . (1996). Phylogenetic diversity of soda lake alkaliphiles. FEMS Microbiol Ecol 19: 181–191.
Foti M, Sorokin DY, Lomans B, Mussman M, Zakharova EE, Pimenov NV et al. (2007). Diversity, activity and abundance of sulfate-reducing bacteria in saline and hypersaline soda lakes. Appl Environ Microbiol 73: 2093–2100.
Fredriksson K, Dube A, Milton DJ, Balasundaram MS . (1973). Lonar Lake, India: an impact crater in basalt. Science 180: 862–864.
Gebre-Mariam Z . (1998). Human interactions and water quality in the Horn of Africa. In: Schoneboom J (eds). Science in Africa- Emerging Water Management Problems. A Publication of the Symposium at the American Association for the Advancement of Science (AAAS), Annual Meeting 1998: Philadelphia, pp 47–61.
Giri BJ, Bano N, Hollibaugh JT . (2004). Distribution of RuBisco genotypes along a redox gradient in Mono Lake, California. Appl Environ Microbiol 70: 3443–3448.
Grant WD, Jones BE, Mwatha WE . (1990). Alkaliphiles: ecology, diversity and applications. FEMS Microbiol Rev 75: 255–270.
Grant WD . (1992). Alkaline environments. In: Lederberg J (ed.) Encyclopaedia of Microbiology 1st edn. Academic Publishers: London, pp. 73–80.
Grant S, Grant WD, Jones BE, Kato C, Li L . (1999). Novel archaeal phytotypes from an East African alkaline saltern. Extremophiles 3: 139–145.
Grant WD . (2006). Alkaline environments and biodiversity, in extremophilies. In: Gerday Charles, Glansdorff Nicolas (eds). Encyclopedia of Life Support Systems (EOLSS). Developed under the Auspices of the UNESCO, Eolss Publishers: Oxford, UK, http://www.eolss.net.
Grant WD, Heaphy S . (2010). Metagenomics and recovery of enzyme genes from alkaline saline environments. Environ Technol 31: 1135–1143.
Grant WD, Sorokin DY . (2011). Distribution and diversity of soda lake alkaliphiles. In: Horikoshi K, Antranikian G, Bull AT, Robb FT, Stetter KO (eds). Extremophiles Handbook vol. 1. Springer: Tokyo, pp. 27–54.
Hoeft SE, Kulp TR, Stolz JF, Hollibaugh JT, Oremland RS . (2004). Dissimilatory arsenate reduction with sulfide as electron donor: Experiments with Mono Lake water and isolation of strain MLMS-1, a chemoautotrophic arsenate-respirer. Appl Env Microb 70: 2741–2747.
Hoeft SE, Blum JS, Stolz JF, Tabita FR, Witte B, King GM et al. (2007). Alkalilimnicola ehrlichii sp. nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor. Int J Syst Evol Microbiol 57: 504–512.
Humayoun SB, Bano N, Hollibaugh JT . (2003). Depth distribution of microbial diversity in Mono Lake, a meromictic soda lake in California. Appl Environ Microbiol 69: 1030–1042.
Hunger S, Schmidt O, Hilgarth M, Horn MA, Kolb S, Conrad R et al. (2011). Competing formate- and carbon-dioxide utilizing prokaryotes in an anoxic methane-emitting fen soil. Appl Environ Microbiol 77: 3773–3785.
Ito S, Kobayashi T, Hatada Y, Horikoshi K . (2005). Enzymes in modern detergents. In: Barredo JL (eds). Microbial Enzymes and Biotransformations. Humana Press: Totowa, NJ, pp. 151–164.
Iversen N, Oremland RS, Klug MJ . (1987). Big Soda Lake (Nevada). 3. Pelagic methanogenesis and anaerobic methane oxidation. Limnol Oceanogr 32: 804–814.
Jones BE, Grant WD, Duckworth AW, Owenson GG . (1998). Microbial diversity of soda lakes. Extremophiles 2: 191–200.
Joshi AA, Kanekar PP, Kelkar AS, Shouche YS, Vani AA, Borgave SB, Sarnaik SS . (2008). Cultivable bacterial diversity of alkaline Lonar Lake, India. Microb Ecol 55: 163–172.
Jourdan FF, Moynier F, Koeberl C, Eroglu S . (2011). 40Ar/39Ar age of the Lonar crater and consequence for the geochronology of planetary impacts. Geology 39: 671–674.
Kaluzhnaya M, Khmelenina V, Eshinimaev B, Suzina N, Nikitin D, Solonin A et al. (2001). Taxonomic characterization of new alkaliphilic and alkalitolerant methanotrophs from soda lakes of the Southeastern Transbaikal region and description of Methylomicrobium buryatense sp. nov. Syst Appl Microbiol 24: 166–176.
Karpeta WP . (1989). Bedded cherts in the Rietgat Formation, Hartbeesfontain, South Africa: a late Archean to early Proterozoic magadiitic alkaline playa lake deposit? South Afr J Geol 92: 29–36.
Khmelenina VN, Kalyuzhnaya MG, Starostina NG, Suzina NE, Trotsenko YA . (1997). Isolation and characterization of halotolerant alkaliphilic methanotrophic bacteria from Tuva soda lakes. Curr Microbiol 35: 257–261.
Khmelenina VN, Eshinimaev BT, Kalyuzhnaya MG, Trotsenko I . (2000). Potential activity of methane and ammonia oxidation by methanotropic communities from soda lakes of the southern Transbaikal. Mikrobiologiia 69: 553–558.
Kovaleva OL, Tourova TP, Muyzer G, Kolganova TV, Sorokin DY . (2011). Diversity of RuBisco and ATP citrate lyase genes in soda lake sediments. FEMS Microbiol Ecol 75: 37–47.
Kumar PA, Srinivas TNR, Kumar PP, Madhu S, Shivaji S . (2010a). Nitritalea halalkaliphila gen. nov., sp. nov., an alkaliphilic bacterium of the family ‘Cyclobacteriaceae’, phylum Bacteroidetes. Int J Syst Evol Microbiol 60: 2320–2325.
Kumar PA, Srinivas TNR, Madhu S, Manorama R, Shivaji S . (2010b). Indibacter alkaliphilus gen. nov., sp. nov., an alkaliphilic bacterium isolated from a haloalkaline lake. Int J Syst Evol Microbiol 60: 721–726.
Kumar PA, Srinivas TNR, Madhu S, Sravan R, Singh S, Naqvi SWA et al. (2012). Cecembia lonarensis gen. nov., sp. nov., a novel haloalkalitolerant bacterium of the family ‘Cyclobacteriaceae’, isolated from a haloalkaline lake and emended descriptions of the genera Indibacter, Nitritalea and Belliella. Int J Syst Evol Microbiol 62: 2252–2258.
LeCleir GR, Buchan A, Maurer J, Moran MA, Hollibaugh JT . (2007). Comparison of chitinolytic enzymes from an alkaline, hypersaline lake and an estuary. Environ Microb 9: 197–205.
Lin JL, Radajewski S, Eshinimaev BT, Trotsenko YA, McDonald IR, Murrell JC . (2004). Molecular diversity of methanotrophs in Transbaikal soda lake sediments and identification of potentially active populations by stable isotope probing. Environ Microbiol 6: 1049–1060.
Lin JL, Joye SB, Scholten JC, Schafer H, McDonald IR, Murrell JC . (2005). Analysis of methane monooxygenase genes in Mono lake suggests that increased methane oxidation activity may correlate with a change in methanotroph community structure. Appl Environ Microbiol 71: 6458–6462.
Linhoff BS, Bennett PC, Puntsag T, Gerel O . (2011). Geochemical evolution of uraniferous soda lakes in Eastern Mongolia. Environ Earth Sci 62: 171–183.
Maloof AC, Stewart ST, Weiss BP, Soule SA, Swanson-Hysell NL, Louzada KL et al. (2010). Geology of Lonar Crater, India. Geol Soc Am Bull 122: 109–126.
McInerney MJ, Bryant MP . (1981). Basic principles of bioconversions in anaerobic digestion and methanogenesis. In: Sofer S, Zaborsky OR (eds). Biomass Conversion Processes for Energy and Fuels. Plenum Press: New York, NY, pp. 277–296.
McInerney MJ, Struchtemeyer CG, Sieber J, Mouttaki H, Stams AJM, Schink B et al. (2008). Physiology, ecology, phylogeny, and genomics of microorganisms capable of syntrophic metabolism. In: Wiegel J, Maier RJ, Adams MWW (eds). Incredible Anaerobes: From Physiology to Genomics to Fuels. New York Academy of Sciences: Boston, MA, pp. 58–72.
Melack JM, Kilham P . (1974). Photosynthetic rates of phytoplankton in East African alkaline, saline lakes. Limnol Oceanogr 19: 743–755.
Moussard H, Smith TJ, Murrell JC . (2011). DNA-stable isotope probing and gene mining. In: Murrell JC, Whiteley A (eds). Stable Isotope Probing and Related Technologies. ASM press: Washington, DC, 3–24.
Neufeld JD, Murrell JC . (2007). Witnessing the last supper of uncultivated microbial cells with Raman-FISH. ISME J 4: 269–270.
Oremland RS, Marsh L, Des Marais DJ . (1982). Methanogenesis in Big Soda Lake, Nevada: an alkaline, moderately hypersaline desert lake. Appl Environ Microbiol 43: 462–468.
Oremland RS, Cloern JE, Sofer Z, Smith RL, Culbertson CW, Zehr J et al. (1988). Microbial and biogeochemical processes in Big Soda Lake, Nevada. In: Fleet AJ, Kelts K, Talbot MR (eds). Lacustrine Petroleum Source Rocks. Geological Society Special Publication: London, No. 40: pp. 59–75.
Oremland RS . (1990). Nitrogen fixation dynamics of two diazotrophic communities in Mono Lake, California. Appl Environ Microbiol 56: 614–622.
Oremland RS, Miller LG, Culbertson CW, Robinson S, Smith RL, Lovley DR et al. (1993). Aspects of the biogeochemistry of methane in Mono Lake and the Mono Basin of California, USA. In: Oremland RS (ed.) The Biogeochemistry of Global Change: Radiative Trace Gases. Chapman & Hall: New York, pp. 704–744.
Oremland RS, Stolz JF, Hollibaugh JT . (2004). The microbial arsenic cycle in Mono Lake, California. FEMS Microb Ecol 48: 15–27.
Oremland RS, Kulp TR, Switzer Blum J, Hoeft SE, Baesman S, Miller LG, Stolz JF . (2005). A microbial arsenic cycle in a salt saturated, βextreme environment. Science 308: 1305–1308.
Pastor JM, Salvador M, Argandona M, Bernal V, Reina-Bueno M, Csonka LN et al. (2010). Ectoines in cell stress protection: uses and biotechnological production. Biotechnol Adv 28: 782–801.
Rees HC, Grant WD, Jones BE, Heaphy S . (2004). Diversity of Kenyan soda lake alkaliphiles assessed by molecular methods. Extremophiles 8: 63–71.
Sheridan C . (2004). Kenyan dispute illuminates bioprospecting difficulties. Nat Biotechnol 22: 1337.
Sorokin DY . (1998). Occurrence of nitrification in extremely alkaline environments. Microbiology (Moscow, English translation) 67: 335–339.
Sorokin DY, Muyzer G, Brinkhoff T, Kuenen JG, Jetten M . (1998). Isolation and characterization of a novel facultatively alkaliphilic Nitrobacter species–Nb. alkalicus. Arch Microbiol 170: 345–352.
Sorokin DY, Jones BE, Kuenen JG . (2000a). A novel obligately methylotrophic, methane-oxidizing Methylomicrobium species from a highly alkaline environment. Extremophiles 4: 145–155.
Sorokin DY, Robertson LA, Kuenen JG . (2000b). Isolation and characterization of obligately chemolithoautotrophic alkaliphilic sulfur-oxidizing bacteria. Ant V Leeuwenhoek 77: 251–260.
Sorokin D, Tourova T, Schmid MC, Wagner M, Koops H, Kuenen JG, Jetten M . (2001). Isolation and properties of obligately chemolithoautotrophic and extremely alkali-tolerant ammonia-oxidizing bacteria from Mongolian soda lakes. Arch Microbiol 176: 170–177.
Sorokin DY, Antipov AN, Kuenen JG . (2003). Complete denitrification in coculture of obligately chemolithoautotrophic haloalkaliphilic sulfur-oxidizing bacteria from a hypersaline soda lake. Arch Microbiol 180: 127–133.
Sorokin DY, Gorlenko VM, Namsaraev BB, Namsaraev ZB, Lysenko AM, Eshinimaev BT et al. (2004). Prokaryotic communities of the north-eastern Mongolian soda lakes. Hydrobiologia 522: 235–248.
Sorokin DY, Foti M, Pinkart HC, Muyzer G . (2007). Sulfur-oxidizing bacteria in Soap Lake (Washington, USA), a meromictic, haloalkaline lake with an unprecedented high sulfide content. Appl Environ Microbiol 73: 451–455.
Sorokin DY, Kuenen JG, Muyzer G . (2011). The microbial sulfur cycle at extremely haloalkaline conditions of soda lakes. Front Microbiol 2: 44.
Srinivas A, Rahul K, Sasikala Ch, Subhash Y, Ramaprasad EV, Ramana ChV . (2012). Georgenia satyanarayanai sp. nov., an alkaliphilic and thermotolerant amylase producing actinobacterium isolated from a soda lake. Int J Syst Evol Microbiol 62: 2405–2409.
Surakasi VP, Wani AA, Shouche YS, Ranade DR . (2007). Phylogenetic analysis of methanogenic enrichment cultures obtained from Lonar Lake in India: isolation of Methanocalculus sp. and Methanoculleus sp. Microb Ecol 54: 697–704.
Surakasi VP, Antony CP, Sharma S, Patole MS, Shouche YS . (2010). Temporal bacterial diversity and detection of putative methanotrophs in surface mats of Lonar crater lake. J Basic Microbiol 50: 465–474.
Switzer Blum J, Burns Bindi A, Buzzelli J, Stolz JF, Oremland RS . (1998). Bacillus arsenicoselenatis, sp. nov., and Bacillus selenitireducens sp. nov: two haloalkaliphiles from Mono Lake, California, that respire oxyanions of selenium and arsenic. Arch Microbiol 171: 19–30.
Thakker CD, Ranade DR . (2002). Alkalophilic Methanosarcina isolated from Lonar Lake. Curr Sci 82: 455–458.
Trotsenko YA, Khmelenina VN . (2002). Biology of extremophilic and extremotolerant methanotrophs. Arch Microbiol 177: 123–131.
Wani AA, Surakasi VP, Siddharth J, Raghavan RG, Patole MS, Ranade D, Shouche YS . (2006). Molecular analyses of microbial diversity associated with the Lonar soda lake in India: an impact crater in a basalt area. Res Microbiol 157: 928–937.
Ward BB, Martino DP, Diaz MC, Joye SB . (2000). Analysis of ammonia-oxidizing bacteria from a hypersaline Mono Lake, California, on the basis of 16S rRNA sequences. Appl Environ Microbiol 66: 2873–2881.
Wüst PK, Horn MA, Drake HL . (2009). Trophic links between fermenters and methanogens in a moderately acidic fen soil. Environ Microbiol 11: 1395–1409.
Zavarzin GA, Zhilina TN, Pusheva MA . (1994). Halophilic acetogenic bacteria. In: Drake HL (eds). Acetogenesis. Chapman & Hall: New York, pp 432–444.
Zavarzin GA, Zhilina TN, Kevbrin VV . (1999). The alkaliphilic microbial community and its functional diversity. Microbiologiya 68: 503–521.
Acknowledgements
This work received financial support from the British Council UK-India Education and Research Initiative Grant SA07-061. CPA acknowledges a senior research fellowship from the Indian Council of Medical Research, Government of India. DK acknowledges an OCE postdoctoral fellowship from CSIRO, Australia.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Paul Antony, C., Kumaresan, D., Hunger, S. et al. Microbiology of Lonar Lake and other soda lakes. ISME J 7, 468–476 (2013). https://doi.org/10.1038/ismej.2012.137
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2012.137
Keywords
This article is cited by
-
Identification of environmental stress parameters to study the natural colour change of water in highly saline inland Crater Lake at Lonar, India
Environmental Monitoring and Assessment (2023)
-
Microbial diversity in extreme environments
Nature Reviews Microbiology (2022)
-
Lonar Impact Crater, India: the Best-Preserved Terrestrial Hypervelocity Impact Crater in a Basaltic Terrain as a Potential Global Geopark
Geoheritage (2022)
-
Grazing pressure-induced shift in planktonic bacterial communities with the dominance of acIII-A1 actinobacterial lineage in soda pans
Scientific Reports (2020)
-
Exploring the upper pH limits of nitrite oxidation: diversity, ecophysiology, and adaptive traits of haloalkalitolerant Nitrospira
The ISME Journal (2020)