Abstract
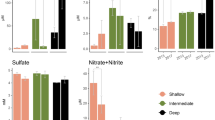
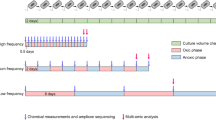
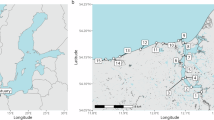
Although microorganisms largely drive many ecosystem processes, the relationship between microbial composition and their functioning remains unclear. To tease apart the effects of composition and the environment directly, microbial composition must be manipulated and maintained, ideally in a natural ecosystem. In this study, we aimed to test whether variability in microbial composition affects functional processes in a field setting, by reciprocally transplanting riverbed sediments between low- and high-salinity locations along the Nonesuch River (Maine, USA). We placed the sediments into microbial ‘cages’ to prevent the migration of microorganisms, while allowing the sediments to experience the abiotic conditions of the surroundings. We performed two experiments, short- (1 week) and long-term (7 weeks) reciprocal transplants, after which we assayed a variety of functional processes in the cages. In both experiments, we examined the composition of bacteria generally (targeting the 16S rDNA gene) and sulfate-reducing bacteria (SRB) specifically (targeting the dsrAB gene) using terminal restriction fragment length polymorphism (T-RFLP). In the short-term experiment, sediment processes (CO2 production, CH4 flux, nitrification and enzyme activities) depended on both the sediment’s origin (reflecting differences in microbial composition between salt and freshwater sediments) and the surrounding environment. In the long-term experiment, general bacterial composition (but not SRB composition) shifted in response to their new environment, and this composition was significantly correlated with sediment functioning. Further, sediment origin had a diminished effect, relative to the short-term experiment, on sediment processes. Overall, this study provides direct evidence that microbial composition directly affects functional processes in these sediments.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abdo Z, Schüette UME, Bent SJ, Williams CJ, Forney LJ, Joyce P . (2006). Statistical methods for characterizing diversity of microbial communities by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Environ Microbiol 8: 929–938.
Allison SD, Lu Y, Weihe C, Goulden ML, Martiny AC, Treseder KK et al (in press). Microbial abundance and composition influence litter decomposition response to environmental change. Ecology (http://dx.doi.org/10.1890/12-1243.1).
Allison SD, Martiny JBH . (2008). Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA 105: 11512–11519.
Austin AT, Sala OE, Jackson RB . (2007). Inhibition of nitrification alters carbon turnover in the patagonian steppe. Ecosystems 9: 1257–1265.
Avrahami S, Conrad R, Braker G . (2002). Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers. Appl Environ Microbiol 68: 5685–5692.
Balser TC, Firestone MK . (2005). Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 73: 395–415.
Bell T, Newman J, Silverman B, Turner S, Lilley A . (2005). The contribution of species richness and composition to bacterial services. Nature 436: 1157–1160.
Berga M, Székely AJ, Langenheder S . (2012). Effects of disturbance intensity and frequency on bacterial community composition and function. PLoS One 7: e36959.
Bernhard A, Tucker J, Giblin A, Stahl D . (2007a). Functionally distinct communities of ammonia-oxidizing bacteria along an estuarine salinity gradient. Environ Microbiol 9: 1439–1447.
Bernhard AE, Tucker J, Giblin AE, Stahl DA . (2007b). Functionally distinct communities of ammonia-oxidizing bacteria along an estuarine salinity gradient. Environ Microbiol 9: 1439–1447.
Bertilsson S, Eiler A, Nordqvist A, Jørgensen NOG . (2007). Links between bacterial production, amino-acid utilization and community composition in productive lakes. ISME J 1: 532–544.
Bottomley P, Taylor A, Boyle S, Mcmahon S, Rich J, Cromack K et al (2004). Responses of nitrification and ammonia-oxidizing bacteria to reciprocal transfers of soil between adjacent coniferous forest and meadow vegetation in the Cascade Mountains of Oregon. Microb Ecol 48: 500–508.
Cavigelli MA, Robertson GP . (2000). The functional significance of denitrifier community composition in a terrestrial ecosystem. Ecology 81: 1402–1414.
Clarke KR, Warwick RM . (2001) Change in Marine Communities: An Approach to Statistical Analysis and Interpretation 2nd edn. PRIMER-E: Plymouth, UK.
Comte J, del Giorgio PA . (2010). Linking the patterns of change in composition and function in bacterioplankton successions along environmental gradients. Ecology 91: 1466–1476.
Comte J, del Giorgio PA . (2011). Composition influences the pathway but not the outcome of the metabolic response of bacterioplankton to resource shifts. PLoS One 6: e25266.
Crain CM . (2007). Shifting nutrient limitation and eutrophication effects in marsh vegetation across estuarine salinity gradients. Estuaries Coasts 30: 26–34.
Daleo P, Fanjul E, Casariego AM, Silliman BR, Bertness MD, Iribarne O . (2007). Ecosystem engineers activate mycorrhizal mutualism in salt marshes. Ecol Lett 10: 902–908.
Dantas G, Sommer MOA, Oluwasegun RD, Church GM . (2008). Bacteria subsisting on antibiotics. Science 320: 100–103.
Dimitriu PA, Lee D, Grayston SJ . (2010). An evaluation of the functional significance of peat microorganisms using a reciprocal transplant approach. Soil Biol Biochem 42: 65–71.
Doane TA, Horwath WR . (2003). Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36: 2713–2722.
Elliott ET, Heil JW, Kelly EF, Monger HC . (1999). Soil structural and other physical properties. In: Robertson GP, Coleman DC, (eds). Standard Soil Methods for Long-Term Ecological Research. Oxford University Press: New York, NY, pp 74–87.
Ewanchuck PJ, Bertness MD . (2004). Structure and organization of a northern New England salt marsh plant community. J Ecol 92: 72–85.
Franklin RB, Mills AL . (2006). Structural and functional responses of a sewage microbial community to dilution-induced reductions in diversity. Microb Ecol 52: 280–288.
Freitag TE, Chang L, Prosser JI . (2006). Changes in the community structure and activity of betaproteobacterial ammonia-oxidizing sediment bacteria along a freshwater-marine gradient. Environ Microbiol 2006: 684–696.
Fuhrman JA, Hewson I, Schwalbach MS, Steele JA, Brown MV, Naeem S . (2006). Annually reoccurring bacterial communities are predictable from ocean conditions. P Natl Acad Sci USA 103: 13104–13109.
Gasol JM, Comerma M, Garcia JC, Armengol J, Casamayor EO, Kojecka P et al (2002). A transplant experiment to identify the factors controlling bacterial abundance, activity, production, and community composition in a eutrophic canyon-shaped reservoir. Limnol Oceanogr 47: 62–77.
Gilbert JA, Steele JA, Caporaso JG, Steinbrück L, Reeder J, Temperton B et al (2012). Defining seasonal marine microbial community dynamics. ISME J 6: 298–308.
Griffiths B, Ritz K, Wheatley R, Kuan H, Boag B, Christensen S et al (2001). An examination of the biodiversity-ecosystem function relationship in arable soil microbial communities. Soil Biol Biochem 33: 1713–1722.
Hanson CA, Horner-Devine MC, Fuhrman JA, Martiny JBH . (2012). Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol 10: 497–506.
Hart SC, Stark JM, Davidson EA, Firestone MK . (1994). Nitrogen mineralization, immobilization, and nitrification. In: Weaver RW, Angle JS, Bottomley PS, (eds). Methods of Soil Analysis. Part 2. Microbiology and Biochemical Properties. Soil Science Society of America: Madison: Wisconsin, pp 985–1018.
Hartnett D, Wilson G . (1999). Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80: 1187–1195.
Hawkins RJ, Purdy KJ . (2007). Genotypic distribution of an indigenous model microorganism along an estuarine gradient. FEMS Microbiol Ecol 62: 187–194.
Lane DJ . (1991). 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, (eds) Nucleic Acid Techniques in Bacterial Systematics. John Wiley & Sons: Chichester, England, pp 115–147.
Langenheder S, Lindstrom E, Tranvik L . (2006). Structure and function of bacterial communities emerging from different sources under identical conditions. Appl Environ Microbiol 72: 212–220.
Langenheder S, Lindstrom ES, Tranvik LJ . (2005). Weak coupling between community composition and functioning of aquatic bacteria. Limnol Oceanogr 50: 957–967.
Lozupone C, Knight R . (2005). UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71: 8228–8235.
Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL et al (2006). Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4: 102–112.
Mohamed DJ, Martiny JBH . (2010). Patterns of fungal diversity and composition along a salinity gradient. ISME J 5: 379–388.
Odum WE . (1988). Comparative ecology of tidal fresh-water and salt marshes. Annu Rev Ecol Syst 19: 147–176.
Power ME . (1990). Effects of fish in river food webs. Science 250: 811–814.
Reed HE, Martiny JBH . (2007). Testing the functional significance of microbial composition in natural communities. FEMS Microbiol Ecol 62: 161–170.
Saiya-Cork K, Sinsabaugh R, Zak D . (2002). The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34: 1309–1315.
Santillano D, Boetius A, Ramette A . (2010). Improved dsrA-based terminal restriction fragment length polymorphism analysis of sulfate-reducing bacteria. Appl Environ Microbiol 76: 5308–5311.
Schimel J, Balser TC, Wallenstein M . (2007). Microbiol stress-response physiology and its implications for ecosystem function. Ecology 88: 1386–1394.
Schimel J . (1995). Ecosystem consequences of microbial diversity and community structure. In: Chapin FS, Koerner C, (eds). Arctic and Alpine Biodiversity: Patterns, Causes, and Ecosystem Consequences. Springer Verlag: New York, pp 239–254.
Schimel JP, Gulledge J . (1998). Microbial community structure and global trace gases. Global Change Biol 4: 745–758.
Senior E, Lindström EB, Banat IM, Nedwell DB . (1982). Sulfate reduction and methanogenesis in the sediment of a saltmarsh on the east coast of the United Kingdom. Appl Environ Microbiol 43: 987–996.
Singh BK, Bardgett RD, Smith P, Reay DS . (2010). Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol 8: 779–790.
Strickland MS, Lauber C, Fierer N, Bradford MA . (2009). Testing the functional significance of microbial community composition. Ecology 90: 441–451.
Tilman D, Wedin D, Knops J . (1996). Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379: 718–720.
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T et al (1998). Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396: 69–72.
Wagner M, Roger A, Flax J, Brusseau G, DA S . (1998). Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J Bacteriol 180: 2975–2982.
Waldrop MP, Firestone MK . (2006). Response of microbial community composition and function to soil climate change. Microb Ecol 52: 716–724.
Weatherburn MW . (1967). Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39: 971–974.
Wertz S, Degrange V, Prosser JI, Poly F, Commeaux C, Freitag T et al (2006). Maintenance of soil functioning following erosion of microbial diversity. Environ Microbiol 8: 2162–2169.
Acknowledgements
We thank Caryl Ann Becerra, Teresa Conneely, Vicente Gomez-Alvarez, Marie Hennelly, Melissa Lage, Jay Lennon, Faye Lemieux, Adam Martiny, Kristin Matulich, Devon Mohamed, Klaus Nüsslein, Nicole Shapiro and Claudia Weihe for discussions, paper comments, and field and lab assistance. This research was supported by the National Science Foundation (MCB-0701494) and the Gordon and Betty Moore Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Reed, H., Martiny, J. Microbial composition affects the functioning of estuarine sediments. ISME J 7, 868–879 (2013). https://doi.org/10.1038/ismej.2012.154
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2012.154
Keywords
This article is cited by
-
Polychaete Bioturbation Alters the Taxonomic Structure, Co-occurrence Network, and Functional Groups of Bacterial Communities in the Intertidal Flat
Microbial Ecology (2023)
-
Microbial Community Succession Along a Chronosequence in Constructed Salt Marsh Soils
Microbial Ecology (2023)
-
Effect of reclaimed water recharge on bacterial community composition and function in the sediment of the Chaobai River, China
Journal of Soils and Sediments (2023)
-
Long-term nitrogen deposition enhances microbial capacities in soil carbon stabilization but reduces network complexity
Microbiome (2022)
-
Integrating hydrochemical and microbial methods to identify hydraulic connections between three saltworks in southern Laizhou Bay, China
Hydrogeology Journal (2022)