Abstract
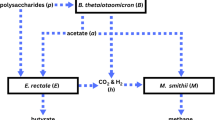
The human gut is colonized by a complex microbiota with multiple benefits. Although the surface-attached, mucosal microbiota has a unique composition and potential to influence human health, it remains difficult to study in vivo. Therefore, we performed an in-depth microbial characterization (human intestinal tract chip (HITChip)) of a recently developed dynamic in vitro gut model, which simulates both luminal and mucosal gut microbes (mucosal-simulator of human intestinal microbial ecosystem (M-SHIME)). Inter-individual differences among human subjects were confirmed and microbial patterns unique for each individual were preserved in vitro. Furthermore, in correspondence with in vivo studies, Bacteroidetes and Proteobacteria were enriched in the luminal content while Firmicutes rather colonized the mucin layer, with Clostridium cluster XIVa accounting for almost 60% of the mucin-adhered microbiota. Of the many acetate and/or lactate-converting butyrate producers within this cluster, Roseburia intestinalis and Eubacterium rectale most specifically colonized mucins. These 16S rRNA gene-based results were confirmed at a functional level as butyryl-CoA:acetate-CoA transferase gene sequences belonged to different species in the luminal as opposed to the mucin-adhered microbiota, with Roseburia species governing the mucosal butyrate production. Correspondingly, the simulated mucosal environment induced a shift from acetate towards butyrate. As not only inter-individual differences were preserved but also because compared with conventional models, washout of relevant mucin-adhered microbes was avoided, simulating the mucosal gut microbiota represents a breakthrough in modeling and mechanistically studying the human intestinal microbiome in health and disease. Finally, as mucosal butyrate producers produce butyrate close to the epithelium, they may enhance butyrate bioavailability, which could be useful in treating diseases, such as inflammatory bowel disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Allison C, McFarlan C, Macfarlane GT . (1989). Studies on mixed populations of human intestinal bacteria grown in single-stage and multistage continuous culture systems. Appl Environ Microbiol 55: 672–678.
Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI . (2005). Host-bacterial mutualism in the human intestine. Science 307: 1915–1920.
Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE et al. (2006). Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol 72: 3593–3599.
Belzer C, de Vos WM . (2012). Microbes inside—from diversity to function: the case of Akkermansia. ISME J 6 (8): 1449–1458.
Boon N, Top EM, Verstraete W, Siciliano SD . (2003). Bioaugmentation as a tool to protect the structure and function of an activated-sludge microbial community against a 3-chloroaniline shock load. Appl Environ Microbiol 69: 1511–1520.
Claesson MJ, Cusack Sn, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E et al. (2011). Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 108: 4586–4591.
Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M . (2000). Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut 47: 589–594.
De Weirdt R, Possemiers S, Vermeulen G, Moerdijk-Poortvliet TCW, Boschker HTS, Verstraete W et al. (2010). Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol Ecol 74: 601–611.
Derrien M, Vaughan EE, Plugge CM, de Vos WM . (2004). Akkermansia muciniphila gen. nov., sp nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54: 1469–1476.
Duncan SH, Hold GL, Barcenilla A, Stewart CS, Flint HJ . (2002). Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int J Syst Evol Microbiol 52: 1615–1620.
Duncan SH, Louis P, Thomson JM, Flint HJ . (2009). The role of pH in determining the species composition of the human colonic microbiota. Environ Microbiol 11: 2112–2122.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M et al. (2005). Diversity of the human intestinal microbial flora. Science 308: 1635–1638.
Falony G, Vlachou A, Verbrugghe K, Vuyst LD . (2006). Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol 72: 7835–7841.
Frank DN, Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR . (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785.
Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ et al. (2009). Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol 3: 148–158.
Hong P-Y, Croix JA, Greenberg E, Gaskins HR, Mackie RI . (2011). Pyrosequencing-based analysis of the mucosal microbiota in healthy individuals reveals ubiquitous bacterial groups and micro-heterogeneity. PLoS ONE 6: e25042.
Jonkers D, Zoetendal EG, Romberg M, Hesselink M, de Vos W, Masclee A et al. (2009). Phylogenetic fingerprinting of the faecal microbiota in IBD-patients using the human intestinal tract (HIT) chip approach: a Twin Study. Gastroenterol 136: A21–A21.
Jumel K, Fiebrig I, Harding SE . (1996). Rapid size distribution and purity analysis of gastric mucus glycoproteins by size exclusion chromatography multi-angle laser light scattering. Int J Biol Macromol 18: 133–139.
Koropatkin NM, Cameron EA, Martens EC . (2012). How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10: 323–335.
Leitch ECM, Walker AW, Duncan SH, Holtrop G, Flint HJ . (2007). Selective colonization of insoluble substrates by human faecal bacteria. Environ Microbiol 9: 667–679.
Lievin-Le Moal V, Servin AL . (2006). The front line of enteric host defense against unwelcome iIntrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev 19: 315–337.
Louis P, Flint HJ . (2007). Development of a semiquantitative degenerate real-time PCR-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol 73: 2009–2012.
Louis P, Flint HJ . (2009). Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294: 1–8.
Louis P, Young P, Holtrop G, Flint HJ . (2010). Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol 12: 304–314.
Macfarlane GT, Macfarlane S, Gibson GR . (1998). Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb Ecol 35: 180–187.
Mäkeläinen H, Hasselwander O, Rautonen N, Ouwehand AC . (2009). Panose, a new prebiotic candidate. Lett Appl Microbiol 49: 666–672.
Muyzer G, Dewaal EC, Uitterlinden AG . (1993). Profiling of complex microbial-populations by denaturing gradient gel-electrophoresis of polymerase chain reaction-amplified genes-coding for 16S ribosomal-RNA. Appl Environ Microbiol 59: 695–700.
Nava GM, Friedrichsen HJ, Stappenbeck TS . (2011). Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J 5: 627–638.
Nava GM, Carbonero F, Croix JA, Greenberg E, Gaskins HR . (2012). Abundance and diversity of mucosa-associated hydrogenotrophic microbes in the healthy human colon. ISME J 6: 57–70.
Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI et al. (2010). Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 105: 2420–2428.
Possemiers S, Verthe K, Uyttendaele S, Verstraete W . (2004). PCR-DGGE-based quantification of stability of the microbial community in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol 49: 495–507.
Rajilic-Stojanovic M, Heilig H, Molenaar D, Kajander K, Surakka A, Smidt H et al. (2009). Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol 11: 1736–1751.
Rajilić-Stojanović M, Maathuis A, HGHJ Heilig, Venema K, de Vos WM, Smidt H . (2010). Evaluating the microbial diversity of an in vitro model of the human large intestine by phylogenetic microarray analysis. Microbiology 156: 3270–3281.
Reis CA, David L, Correa P, Carneiro F, Cd Bolós, Garcia E et al. (1999). Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res 59: 1003–1007.
Roos S, Jonsson H . (2002). A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 148: 433–442.
Schwiertz A, Hold GL, Duncan SH, Gruhl B, Collins MD, Lawson PA et al. (2002). Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilising, butyrate-producing bacterium from human faeces. Syst Appl Microbiol 25: 46–51.
Shen XJ, Rawls JF, Randall TA, Burcall L, Mpande C, Jenkins N et al. (2010). Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes 1: 138–147.
Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux J-J et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 105: 16731–16736.
Strugala V, Dettmar PW, Pearson JP . (2008). Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn's disease. Int J Clin Pract 62: 762–769.
Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H . (2005). Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43: 3380–3389.
Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S, Doerffel Y . (2008). Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology 135: 568–579.
Ten Bruggencate SJM, Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Katan MB, Van der Meer R . (2004). Dietary fructo-oligosaccharides and inulin decrease resistance of rats to salmonella: protective role of calcium. Gut 53: 530–535.
Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain J-P . (2010). Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis 16: 684–695.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI . (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1131.
Van den Abbeele P, Grootaert C, Marzorati M, Possemiers S, Verstraete W, Gérard P et al. (2010). Microbial community development in a dynamic gut model is reproducible, colon-region specific and selects for Bacteroidetes and Clostridium cluster IX. Appl Environ Microbiol 76: 5237–5246.
Van den Abbeele P, Gerard P, Rabot S, Bruneau A, El Aidy S, Derrien M et al. (2011a). Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ Microbiol 13: 2667–2680.
Van den Abbeele P, Roos S, Eeckhaut V, Marzorati M, Possemiers S, Vanhoecke B et al. (2011b). Incorporation of a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb Biotechnol 5: 106–115.
Van den Abbeele P, Van de Wiele T, Verstraete W, Possemiers S . (2011c). The host selects mucosal and luminal associations of coevolved gut microorganisms: a novel concept. FEMS Microbiol Rev 35: 681–704.
van den Bogert B, de Vos WM, Zoetendal EG, Kleerebezem M . (2011). Microarray analysis and barcoded pyrosequencing provide consistent microbial profiles depending on the source of human intestinal samples. Appl Environ Microbiol 77: 2071–2080.
Vermeiren J, Van den Abbeele P, Laukens D, Vigsnæs LK, De Vos M, Boon N et al. (2012). Decreased colonization of fecal Clostridium coccoides/Eubacterium rectale species from ulcerative colitis patients in an in vitro dynamic gut model with mucin environment. FEMS Microbiol Ecol 79: 685–696.
Walker A, Sanderson J, Churcher C, Parkes G, Hudspith B, Rayment N et al. (2011). High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol 11: 7.
Walker AW, Duncan SH, Harmsen HJM, Holtrop G, Welling GW, Flint HJ . (2008). The species composition of the human intestinal microbiota differs between particle-associated and liquid phase communities. Environ Microbiol 10: 3275–3283.
Wang Y, Antonopoulos D, Zhu X, Harrell L, Hanan I, Alverdy J et al. (2010). Laser capture microdissection and metagenomic analysis of intact mucosa-associated microbial communities of human colon. Appl Environ Microbiol 88: 1333–1342.
Willing B, Halfvarson J, Dicksved J, Rosenquist M, Järnerot G, Engstrand L et al. (2009). Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis 15: 653–660.
Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z et al. (2010). A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139: 1844–1854 e1841.
Zoetendal EG, Akkermans ADL, De Vos WM . (1998). Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol 64: 3854–3859.
Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans ADL, de Vos WM . (2002). Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol 68: 3401–3407.
Acknowledgements
PVdA is a Postdoctoral Fellow from FWO-Vlaanderen (Research Foundation of Flanders, Belgium) and RDW is a PhD student funded by the Special Research Fund (BOF) of Ghent University. This work was partially supported by a GOA (BOF12/GOA/008) project from Ghent University, an SBO project (100016) from the Agency for Innovation by Science and Technology (IWT) and an unrestricted Spinoza Award of the Netherlands Foundation for Scientific Research (NWO) and an Advanced Grant of the European Research Council (to WMdV). Finally, we thank Tim Lacoere (Ghent University) for technical assistance and Petra Louis (University of Aberdeen) for her excellent advise with respect to the construction of the butyryl-CoA:acetate-CoA transferase gene clone library and subsequent analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Van den Abbeele, P., Belzer, C., Goossens, M. et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J 7, 949–961 (2013). https://doi.org/10.1038/ismej.2012.158
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2012.158
Keywords
This article is cited by
-
Bridging gut microbiota composition with extended-spectrum beta-lactamase Enterobacteriales faecal carriage in critically ill patients (microbe cohort study)
Annals of Intensive Care (2023)
-
Effects of melatonin on rumen microorganisms and methane production in dairy cow: results from in vitro and in vivo studies
Microbiome (2023)
-
Gut microbiota as a target in the bone health of livestock and poultry: roles of short-chain fatty acids
Animal Diseases (2023)
-
Dietary fiber during gestation improves lactational feed intake of sows by modulating gut microbiota
Journal of Animal Science and Biotechnology (2023)
-
Gut microbiota in a mouse model of obesity and peripheral neuropathy associated with plasma and nerve lipidomics and nerve transcriptomics
Microbiome (2023)