Abstract
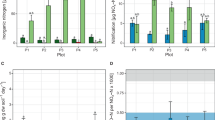
It is well known that the ratio of ammonia-oxidizing archaea (AOA) and bacteria (AOB) ranges widely in soils, but no data exist on what might influence this ratio, its dynamism, or how changes in relative abundance influences the potential contributions of AOA and AOB to soil nitrification. By sampling intensively from cropped-to-fallowed and fallowed-to-cropped phases of a 2-year wheat/fallow cycle, and adjacent uncultivated long-term fallowed land over a 15-month period in 2010 and 2011, evidence was obtained for seasonal and cropping phase effects on the soil nitrification potential (NP), and on the relative contributions of AOA and AOB to the NP that recovers after acetylene inactivation in the presence and absence of bacterial protein synthesis inhibitors. AOB community composition changed significantly (P⩽0.0001) in response to cropping phase, and there were both seasonal and cropping phase effects on the amoA gene copy numbers of AOA and AOB. Our study showed that the AOA:AOB shifts were generated by a combination of different phenomena: an increase in AOA amoA abundance in unfertilized treatments, compared with their AOA counterparts in the N-fertilized treatment; a larger population of AOB under the N-fertilized treatment compared with the AOB community under unfertilized treatments; and better overall persistence of AOA than AOB in the unfertilized treatments. These data illustrate the complexity of the factors that likely influence the relative contributions of AOA and AOB to nitrification under the various combinations of soil conditions and NH4+-availability that exist in the field.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adair KL, Schwartz E . (2008). Evidence that ammonia-oxidizing archaea are more abundant than ammonia-oxidizing bacteria in semiarid soils of northern Arizona, USA. Microbial Ecol 56: 420–426.
Berube PM, Samudrala R, Stahl DA . (2007). Transcription of all amoC copies is associated with recovery of Nitrosomonas europaea from ammonia starvation. J Bacteriol 189: 3935–3944.
Bollmann A, Schmidt I, Saunders AM, Nicolaisen MH . (2005). Influence of starvation on potential ammonia-oxidizing activity and amoA mRNA levels of Nitrosospira briensis. Appl Environ Microbiol 71: 1276–1282.
Boyle-Yarwood SA, Bottomley PJ, Myrold DD . (2008). Community composition of ammonia-oxidizing bacteria and archaea in soils under stands of red alder and Douglas fir in Oregon. Environ Microbiol 10: 2956–2965.
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S et al. (2010). Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72: 386–394.
Hallin S, Jones CM, Schloter M, Philippot L . (2009). Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J 3: 597–605.
He J-Z, Shen J-P, Zhang L-M, Zhu Y-G, Zheng Y-M, Xu M-G et al. (2007). Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9: 3152–3152.
Horz HP, Rotthauwe JH, Lukow T, Liesack W . (2000). Identification of major subgroups of ammonia-oxidizing bacteria in environmental samples by T-RFLP analysis of amoA PCR products. J Microbiol Methods 39: 197–204.
Jia ZJ, Conrad R . (2009). Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11: 1658–1671.
Johnstone BH, Jones RD . (1988a). Physiological effects of long-term energy-source deprivation on the survival of a marine chemolithotrophic ammonium-oxidizing bacterium. Marine Ecol -Progress Series 49: 295–303.
Johnstone BH, Jones RD . (1988b). Recovery of a marine chemolithotrophic ammonium-oxidizing bacterium from long-term energy-source deprivation. Canadian J Microbiol 34: 1347–1350.
Jones RD, Morita RY, Koops HP, Watson SW . (1988). A new marine ammonium-oxidizing bacterium, Nitrosomonas Cryotolerans Sp-Nov. Canadian J Microbiol 34: 1122–1128.
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW et al. (2006). Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442: 806–809.
McCune B, Mefford MJ . (1999), PC-ORD. Multivariate Analysis of Ecological Data. Version 4. MjM Software, Gleneden Beach, Oregon, USA.
Mertens J, Broos K, Wakelin SA, Kowalchuk GA, Springael D, Smolders E . (2009). Bacteria, not archaea, restore nitrification in a zinc-contaminated soil. ISME J 3: 916–923.
Mintie AT, Heichen RS, Cromack K, Myrold DD, Bottomley PJ . (2003). Ammonia-oxidizing bacteria along meadow-to-forest transects in the Oregon cascade mountains. Appl Environ Microbiol 69: 3129–3136.
Norton JM . (2008). Nitrification in agricultural soils. In: Schepers JS, Raun WR (eds) Nitrogen in Agricultural Systems. American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc.: Madison, WI, pp 173–200.
Offre P, Prosser JI, Nicol GW . (2009). Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol Ecol 70: 99–108.
Peterson RG, Calvin LD . (1996). Sampling. In Methods of Soil Analysis, Part 3- Chemical Methods Sparks DL (ed). Soil Science Society of Ameria: Madison, Wisconsin, pp 1–18.
Rotthauwe JH, Witzel KP, Liesack W . (1997). The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63: 4704–4712.
Schauss K, Focks A, Leininger S, Kotzerke A, Heuer H, Thiele-Bruhn S et al. (2009). Dynamics and functional relevance of ammonia-oxidizing archaea in two agricultural soils. Environ Microbiol 11: 446–456.
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ . (2008). Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ Microbiol 10: 1601–1611.
Shi W, Miller BE, Stark JM, Norton JM . (2004). Microbial nitrogen transformations in response to treated dairy waste in agricultural soils. Soil Sci Soc America J 68: 1867–1874.
Taylor AE, Zeglin LH, Dooley S, Myrold DD, Bottomley PJ . (2010). Evidence for different contributions of archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils. Appl Environ Microbiol 76: 7691–7698.
Tourna M, Freitag TE, Nicol GW, Prosser JI . (2008). Growth, activity and temperature responses of ammonia-oxidizing archaea and bacteria in soil microcosms. Environ Microbiol 10: 1357–1364.
Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T et al. (2011). Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA 108: 8420–8425.
Verhamme DT, Prosser JI, Nicol GW . (2011). Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J 5: 1067–1071.
Wessen E, Nyberg K, Jansson JK, Hallin S . (2010). Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl Soil Ecol 45: 193–200.
Wessen E, Soderstrom M, Stenberg M, Bru D, Hellman M, Welsh A et al. (2011). Spatial distribution of ammonia-oxidizing bacteria and archaea across a 44-hectare farm related to ecosystem functioning. ISME J 5: 1213–1225.
Xia W, Zhang C, Zeng X, Feng Y, Weng J, Lin X et al. (2011). Autotrophic growth of nitrifying community in an agricultural soil. ISME J 5: 1226–1236.
Zeglin L, Taylor AE, Bottomley PJ, Myrold DD . (2011). Bacterial and archaeal amoA gene distribution covaries with soil nitrification properties across a range of land uses. Environ Microbiol Reports 3: 717–726.
Zhang LM, Offre PR, He JZ, Verhamme DT, Nicol GW, Prosser JI . (2010). Autotrophic ammonia oxidation by soil thaumarchaea. Proc Natl Acad Sci USA 107: 17240–17245.
Acknowledgements
This research was funded by USDA CSREES Agreement No. 2007-35107-18355. Additional support obtained from the Oregon State University community included: technical services from the Central Analytical Laboratory, QPCR facilities at the Center for Genome Research and Biocomputing, and field sites maintained by the Hyslop Field Research Laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Taylor, A., Zeglin, L., Wanzek, T. et al. Dynamics of ammonia-oxidizing archaea and bacteria populations and contributions to soil nitrification potentials. ISME J 6, 2024–2032 (2012). https://doi.org/10.1038/ismej.2012.51
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2012.51
Keywords
This article is cited by
-
Contribution of ammonia-oxidizing archaea and bacteria to nitrogen transformation in a soil fertilized with urea and organic amendments
Scientific Reports (2023)
-
Nitrification inhibitor 1-octyne inhibits growth of comammox Nitrospira but does not alter their community structure in an acidic soil
Journal of Soils and Sediments (2023)
-
Competition shifts the advantage of the invasive plant Bidens alba to a disadvantage under soil ammonia nitrogen
Biological Invasions (2023)
-
Structure and function of the soil microbiome underlying N2O emissions from global wetlands
Nature Communications (2022)
-
Seasonal dynamics of ammonia-oxidizing bacteria but not archaea influence soil nitrogen cycling in a semi-arid agricultural soil
Scientific Reports (2022)