Abstract
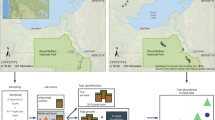
Although recent work has shown that both deterministic and stochastic processes are important in structuring microbial communities, the factors that affect the relative contributions of niche and neutral processes are poorly understood. The macrobiological literature indicates that ecological disturbances can influence assembly processes. Thus, we sampled bacterial communities at 4 and 16 weeks following a wildfire and used null deviation analysis to examine the role that time since disturbance has in community assembly. Fire dramatically altered bacterial community structure and diversity as well as soil chemistry for both time-points. Community structure shifted between 4 and 16 weeks for both burned and unburned communities. Community assembly in burned sites 4 weeks after fire was significantly more stochastic than in unburned sites. After 16 weeks, however, burned communities were significantly less stochastic than unburned communities. Thus, we propose a three-phase model featuring shifts in the relative importance of niche and neutral processes as a function of time since disturbance. Because neutral processes are characterized by a decoupling between environmental parameters and community structure, we hypothesize that a better understanding of community assembly may be important in determining where and when detailed studies of community composition are valuable for predicting ecosystem function.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Oliveros JC (2007), VENNY; An interactive tool for comparing lists with Venn Diagrams http://bioinfogp.cnb.csic.es/tools/venny/index.html.
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL et al (2011). Navigating the multiple meanings of beta-diversity: a roadmap for the practicing ecologist. Ecol Lett 14: 19–28.
Beck T, Joergensen G, Kandeler E, Makeschin F, Nuss E, Oberholzer HR et al (1997). An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29: 1023–1032.
Bell G . (2001). Neutral macroecology. Science 293: 2413–2418.
Bonan GB, Shugart HH . (1989). Environmental factors and ecological processes in boreal forests. Annu Rev Ecol Syst 20: 1–28.
Bond WJ, Woodward FI, Midgley GF . (2005). The global distribution of ecosystems in a world without fire. New Phytol 165: 525–538.
Bray JR, Curtis JT . (1957). An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27: 325–349.
Brookes PC, Landman A, Pruden G, Jenkinson DS . (1985). Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17: 837–842.
Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S . (2011). Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J 5: 590–600.
Bárcenas-Moreno G, García-Orenes F, Mataix-Solera J, Mataix-Beneyto J, Bååth E . (2011). Soil microbial recolonisation after a fire in a Mediterranean forest. Biol Fert Soils 47: 261–272.
Cadotte MW . (2007). Concurrent niche and neutral processes in the competition–colonization model of species coexistence. Proc R Soc B 274: 2739–2744.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger J, Bushman FD, Costello EK et al (2010). QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336.
Caruso T, Chan Y, Lau MCY, McKay CP, Pointing SB . (2011). Stochastic and deterministic processes interact in the assembly of desert microbial communities on a global scale. ISME J 5: 1406–1413.
Certini G . (2005). Effects of fire on properties of forest soils: a review. Oecologia 143: 1–10.
Chase JM, Myers JA . (2011). Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc Lond B Biol Sci 366: 2351–2363.
Chase JM . (2003). Community assembly: when should history matter? Oecologia 136: 489–498.
Chase JM . (2007). Drought mediates the importance of stochastic community assembly. Proc Natl Acad Sci USA 104: 17430–17434.
Chytrý M, Lososová Z, Horsák M, Uher B, Čejka T, Danihelka J et al (2012). Dispersal limitation is stronger in communities of microorganisms than macroorganisms across Central European cities. J Biogeography 39: 1101–1111.
DeLuca TH, Sala A . (2006). Frequent fire alters nitrogen transformations in ponderosa pine stands of the Inland Northwest. Ecology 87: 2511–2522.
Denslow JS . (1980). Gap portioning among tropical rainforest trees. Biotropica 12: 47–55.
Didham RK, Norton DA . (2006). When are alternative stable states more likely to occur? Oikos 113: 357–362.
Didham RK, Watts CH, Norton DA . (2005). Are systems with strong underlying abiotic regimes more likely to exhibit alternative stable states? Oikos 110: 409–416.
Edgar RC . (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461.
Ellner SP, Fussmann G . (2003). Effects of successional dynamics on metapopulation persistence. Ecology 84: 882–889.
Ferrenberg SM, Schwilk DW, Knapp EE, Groth E, Keeley JE . (2006). fire decreases arthropod abundance but increases diversity: early and late season prescribed fire effects in a sierra nevada mixed-conifer forest. Fire Ecol 2: 79–102.
Fierer N, Jackson R . (2006). The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103: 626–631.
Hamman ST, Burke IC, Knapp EE . (2008). Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. Forest Ecol Manag 256: 367–374.
Hanson CA, Fuhrman J, Horner-Devine MC, Martiny JBH . (2012). Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol 10: 497–506.
Hart SC, DeLuca TH, Newman GS, MacKenzie MD, Boyle SI . (2005). Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For Ecol Manage 220: 166–184.
Hubbell SP . (2001) The Unified Neutral Theory Of Biodiversity And Biogeography. Princeton University Press: Princeton, NJ.
Jiang L, Patel SN . (2008). Community assembly in the presence of disturbance: a microcosm experiment. Ecology 89: 1931–1940.
JMP Version 10 (2011). SAS Institute Inc.: Cary, NC.
Knelman JE, Legg TM, O’Neill SP, Washenberger CL, González A, Cleveland CC et al (2012). Bacterial community structure and function change in association with colonizer plants during early primary succession in a glacier forefield. Soil Biol Biochem 46: 172–180.
Kraft NJB, Cornwell WK, Webb CO, Ackerly DD . (2007). Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am Nat 170: 271–283.
Langenheder S, Bulling MT, Solan M, Prosser JI . (2010). Bacterial biodiversity-ecosystem functioning relations are modified by environmental complexity. PloS one 5: 10834.
Langenheder S, Prosser JI . (2008). Resource availability influences the diversity of a functional group of heterotrophic soil bacteria. Environ Microbiol 10: 2245–2256.
Langenheder S, Székely AJ . (2011). Species sorting and neutral processes are both important during the initial assembly of bacterial communities. ISME J 5: 1086–1094.
Leibold MA, McPeek MA . (2006). Coexistence of the niche and neutral perspectives in community ecology. Ecology 8: 1399–1410.
Logue JB, Lindström ES . (2010). Species sorting affects bacterioplankton community composition as determined by 16S rDNA and 16S rRNA fingerprints. ISME J 4: 729–738.
Lozupone C, Knight R . (2005). ‘UniFrac: a new phylogenetic method for comparing microbial communities’. Appl Environ Microbiol 71: 8228–8235.
Lozupone C, Hamady M, Knight R . (2006). UniFrac – an online tool for comparing microbial community diversity in a phylogenetic context. BMC bioinformatics 7: 371.
Lozupone CA, Knight R . (2007). Global patterns in bacterial diversity. Proc Natl Acad Sci USA 104: 11436–11440.
Martiny JBH, Bohannan BJM, Brown JH, Colwell RK, Fuhrman JA, Green JL et al (2006). Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4: 102–112.
McCune B, Mefford MJ . (2011) PC-ORD. Multivariate Analysis of Ecological Data. Version 6.0. MjM Software: Gleneden Beach, OR.
Monson RK, Lipson DL, Burns SP, Turnipseed AA, Delany AC, Williams MK et al (2006). Winter forest soil respiration controlled by climate and microbial community composition. Nature 439: 711–714.
Mulvaney RL . (1996). Nitrogen - Inorganic forms. In: Sparks, DL, et al. (ed) Methods of soil analysis, Part 3, SSSA Book Series 5. SSSA: Madison, WI, pp 1123–1184.
Nemergut DR, Anderson SP, Cleveland CC, Martin AP, Miller AE, Seimon A et al (2007). Microbial community succession in an unvegetated, recently-deglaciated soil. Microb Ecol 53: 110–122.
Nemergut DR, Cleveland CC, Wieder WR, Washenberger CL, Townsend AR . (2010). Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol Biochem 42: 2153–2160.
Nemergut DR, Costello EK, Hamady M, Lozupone C, Jiang L, Schmidt SK et al (2011). Global patterns in the biogeography of bacterial taxa. Environ Microbiol 13: 135–144.
Ofiteru ID, Lunn M, Curtis TP, Wells GF, Criddle CS, Francis CA et al (2010). Combined niche and neutral effects in a microbial wastewater treatment community. Proc Natl Acad Sci USA 107: 15345–15350.
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB et al (2011). Vegan: Community Ecology Package. R package version 2: 0–1.
Östman Ö, Drakare S, Kritzberg ES, Langenheder S, Logue JB, Lindström ES . (2009). Regional invariance among microbial communities. Ecology letters 13: 118–127.
Paul EA, Clark FE . (1996). Soil Microbiology and Biochemistry, 2nd Edition. Academic Press: New York, NY.
Peay KG, Garbelotto M, Bruns TD . (2010). Evidence of dispersal limitation in soil microorganisms: isolation reduces species richness on mycorrhizal tree islands. Ecology 91: 3631–3640.
Peitikäinen J, Hiukka R, Fritze H . (2000). Does short-term heating of forest humus change its properties as a substrate for microbes? Soil Biol Biochem 32: 277–288.
Reeder J, Knight R . (2010). Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat Methods 7: 688–689.
Sattin SR, Cleveland CC, Hood E, Reed SC, King AJ, Schmidt SK et al (2009). Functional shifts in unvegetated, perhumid, recently deglaciated soils do not correlate with shifts in soil bacterial community composition. J Microbiol 47: 673–681.
Schmidt SK, Costello EK, Nemergut DR, Cleveland CC, Reed SC, Weintraub MN et al (2007). Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 88: 1379–1385.
Schoennagel T, Sherriff RL, Veblen TT . (2011). Fire history and tree recruitment in the Colorado Front Range upper montane zone: implications for forest restoration. Ecol Appl 21: 2210–2222.
Sloan WT, Mary Lunn M, Woodcock S, Head IM, Nee S, Curtis TP . (2006). Quantifying the roles of immigration and chance in shaping prokaryote community structure. Env Microbiol 8: 732–740.
Smith NR, Kishchuk BE, Mohn WW . (2008). Effects of wildfire and harvest disturbances on forest soil bacterial communities. Appl Environ Microbiol 74: 216–224.
Team RDC . (2011) R: A Language And Environment For Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria.
Telford RJ, Vandvik V Birks HJB (2006). Dispersal limitation matters for microbial morphospecies. Science 312: 1015.
Turner MG, Smithwick EAH, Metzger KL, Tinker DB, Romme WH . (2007). Inorganic nitrogen availability following severe stand-replacing fire in the Greater Yellowstone Ecosystem. Proc Natl Acad Sci USA 104: 4782–4789.
Van Der Heijden MGA, Bardgett RD, Van Straalen NM . (2008). The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11: 296–310.
Veblen TT, Kitzberger T, Donnegan J . (2000). Climatic and human influences on fire regimes in ponderosa pine forests in the Colorado Front Range. Ecol Appl 10: 1178–1195.
Vellend M . (2010). Conceptual synthesis in community ecology. Q Rev Biol 85: 183–206.
Waldrop MP, Harden JW . (2008). Interactive effects of wildfire and permafrost on microbial communities and soil processes in an Alaskan black spruce forest. Global Change Biol 14: 2591–2602.
Wang GG, Kemball KJ . (2005). Effects of fire severity on early development of understory vegetation. Can J Forest Res 35: 254–262.
Wang Q, Zhong M, Wang S . (2012). A meta-analysis on the response of microbial biomass, dissolved organic matter, respiration, and N mineralization in mineral soil to fire in forest ecosystems. Forest Ecol Manag 271: 91–97.
Wardle DA, Zackrisson O, Hörnberg G, Gallet C . (1997). The influence of island area on ecosystem properties. Science 277: 1296–1299.
Wardle DA, Zackrisson O, Nilsson MC . (1998). The charcoal effect in Boreal forests: mechanisms and ecological consequences. Oecologia 115: 419–426.
Woodcock S, Van Der Gast CJ, Bell T, Lunn M, Curtis TP, Head IM et al (2007). Neutral assembly of bacterial communities. FEMS Microbiol Ecol 62: 171–180.
Yeager CM, Northrup DE, Grow CC, Barns SM, Kuske CR . (2005). Changes in nitrogen-fixing and ammonia-oxidizing bacterial communities in soil of a mixed conifer forest after wildfire. Appl Environ Microbiol 71: 2713–2722.
Yilmaz P, Kottmann R, Field D, Knight R, Cole JR, Amaral-Zettler L et al (2011). Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications. Nat Biotechnol 29: 415–420.
Zak DR, Holmes WE, White DC, Peacock AD, Tilman D . (2003). Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 84: 2042–2050.
Acknowledgements
This research was supported, in part, through grants from the National Science Foundation to DN, SS and CC (DEB1545913), DEB1120281 to LJ and the Niwot Ridge LTER grant (DEB-1027341) to MW, AT, and SS. This research used the Janus supercomputer, which is supported by the National Science Foundation (award number CNS-0821794) and the University of Colorado Boulder. The Janus supercomputer is a joint effort of the University of Colorado Boulder, the University of Colorado Denver and the National Center for Atmospheric Research. We thank Timothy Seastedt for comments on our manuscript and the University of Colorado’s Department of Ecology and Evolutionary Biology for generous support. We acknowledge Bob Head and Bill Nemergut (WFN) for assisting with sample collection and processing. Our manuscript was greatly improved by comments and suggestions from three anonymous reviewers and the editor.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Ferrenberg, S., O'Neill, S., Knelman, J. et al. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J 7, 1102–1111 (2013). https://doi.org/10.1038/ismej.2013.11
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2013.11
Keywords
This article is cited by
-
Elevated methane flux in a tropical peatland post-fire is linked to depth-dependent changes in peat microbiome assembly
npj Biofilms and Microbiomes (2024)
-
Bacterial community network complexity and role of stochasticity decrease during primary succession
Soil Ecology Letters (2024)
-
Implications of Soil Microbial Community Assembly for Ecosystem Restoration: Patterns, Process, and Potential
Microbial Ecology (2023)
-
Assembly Processes and Biogeographical Characteristics of Soil Bacterial Sub-communities of Different Habitats in Urban Green Spaces
Current Microbiology (2023)
-
Forest conversion from pure to mixed Cunninghamia lanceolata plantations enhances soil multifunctionality, stochastic processes, and stability of bacterial networks in subtropical southern China
Plant and Soil (2023)