Abstract
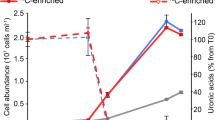
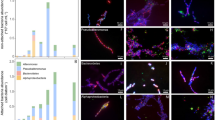
The release of organic material upon algal cell lyses has a key role in structuring bacterial communities and affects the cycling of biolimiting elements in the marine environment. Here we show that already before cell lysis the leakage or excretion of organic matter by infected yet intact algal cells shaped North Sea bacterial community composition and enhanced bacterial substrate assimilation. Infected algal cultures of Phaeocystis globosa grown in coastal North Sea water contained gamma- and alphaproteobacterial phylotypes that were distinct from those in the non-infected control cultures 5 h after infection. The gammaproteobacterial population at this time mainly consisted of Alteromonas sp. cells that were attached to the infected but still intact host cells. Nano-scale secondary-ion mass spectrometry (nanoSIMS) showed ∼20% transfer of organic matter derived from the infected 13C- and 15N-labelled P. globosa cells to Alteromonas sp. cells. Subsequent, viral lysis of P. globosa resulted in the formation of aggregates that were densely colonised by bacteria. Aggregate dissolution was observed after 2 days, which we attribute to bacteriophage-induced lysis of the attached bacteria. Isotope mass spectrometry analysis showed that 40% of the particulate 13C-organic carbon from the infected P. globosa culture was remineralized to dissolved inorganic carbon after 7 days. These findings reveal a novel role of viruses in the leakage or excretion of algal biomass upon infection, which provides an additional ecological niche for specific bacterial populations and potentially redirects carbon availability.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Alderkamp AC, Sintes E, Herndl GJ . (2006). Abundance and activity of major groups of prokaryotic plankton in the coastal North Sea during spring and summer. Aquat Microb Ecol 45: 237–246.
Allers E, Gómez-Consarnau L, Pinhassi J, Gasol JM, Šimek K, Pernthaler J . (2007). Response of Alteromonadaceae and Rhodobacteriaceae to glucose and phosphorus manipulation in marine mesocosms. Environ Microbiol 9: 2417–2429.
Allers E, Niesner C, Wild C, Pernthaler J . (2008). Microbes enriched in seawater after addition of coral mucus. Appl Environ Microbiol 74: 3274–3278.
Amann R, Glöckner FO, Neef A . (1997). Modern methods in subsurface microbiology: in situ identification of microorganisms with nucleic acid probes. Fems Microbiol Rev 20: 191–200.
Assayag N, Rivé K, Ader M, Jézéquel D, Agrinier P . (2006). Improved method for isotopic and quantitative analysis of dissolved inorganic carbon in natural water samples. Rapid Commun Mass Spectrom 20: 2243–2251.
Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F . (1983). The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10: 257–263.
Baudoux AC, Brussaard CPD . (2005). Characterization of different viruses infecting the marine harmful algal bloom species Phaeocystis globosa. Virology 341: 80–90.
Baudoux AC, Noordeloos AAM, Veldhuis MJW, Brussaard CPD . (2006). Virally induced mortality of Phaeocystis globosa during two spring blooms in temperate coastal waters. Aquat Microb Ecol 44: 207–217.
Bell W, Mitchell R . (1972). Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol Bull 143: 265–277.
Brussaard CPD, Gast GJ, vanDuyl FC, Riegman R . (1996). Impact of phytoplankton bloom magnitude on a pelagic microbial food web. Mar Ecol Prog Ser 144: 211–221.
Brussaard CPD . (2004). Optimization of procedures for counting viruses by flow cytometry. Appl Environ Microbiol 70: 1506–1513.
Brussaard CPD, Kuipers B, Veldhuis MJW . (2005a). A mesocosm study of Phaeocystis globosa population dynamics: 1. Regulatory role of viruses in bloom. Harmful Algae 4: 859–874.
Brussaard CPD, Mari X, Van Bleijswijk JDL, Veldhuis MJW . (2005b). A mesocosm study of Phaeocystis globosa (Prymnesiophyceae) population dynamics—II. Significance for the microbial community. Harmful Algae 4: 875–893.
Brussaard CPD, Payet JP, Winter C, Weinbauer MG . (2010). Quantification of aquatic viruses by flow cytometry. In: SW Wilhelm, MG Weinbauer and CA Suttle (eds) Manual of Aquatic Viral Ecology ASLO pp 102–109.
Brussaard CPD, Wilhelm SW, Thingstad F, Weinbauer MG, Bratbak G, Heldal M et al (2008). Global-scale processes with a nanoscale drive: the role of marine viruses. ISME J 2: 575–578.
Cottrell MT, Suttle CA . (1991). Wide-spread occurrence and clonal variation in viruses which cause lysis of a cosmopolitan, eukaryotic marine phytoplankter, Micromonas pusilla. Mar Ecol Prog Ser 78: 1–9.
Edgar RC . (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461.
Eilers H, Pernthaler J, Glockner FO, Amann R . (2000). Culturability and in situ abundance of pelagic bacteria from the North Sea. Applied Environ Microbiol 66: 3044.
Eilers H, Pernthaler J, Peplies J, Glöckner FO, Gerdts G, Amann R . (2001). Isolation of novel pelagic bacteria from the German Bight and their seasonal contributions to surface picoplankton. Appl Environ Microbiol 67: 5134–5142.
Fuchs BM, Zubkov MV, Sahm K, Burkill PH, Amann R . (2000). Changes in community composition during dilution cultures of marine bacterioplankton as assessed by flow cytometric and molecular biological techniques. Environ Microbiol 2: 191–201.
Gómez-Consarnau L, Lindh MV, Gasol JM, Pinhassi J . (2012). Structuring of bacterioplankton communities by specific dissolved organic carbon compounds. Environ Microbiol 14: 2361–2378.
Guillard RRL . (1975). Culture of phytoplankton for feeding marine invertebrates. Smith, Walter L, Chanley Matoira H (eds) Culture of Marine Invertebrate Animals Conference, Greenport, NY Oct, 1972 Viii+338p. Illus Plenum Press: New York, NY, USA; London, England Isbn 0-306-30804-5, 29–60.
Haaber J, Middelboe M . (2009). Viral lysis of Phaeocystis pouchetii: Implications for algal population dynamics and heterotrophic C, N and P cycling. ISME J 3: 430–441.
Huson DH, Mitra S, Ruscheweyh HJ, Weber N, Schuster SC . (2011). Integrative analysis of environmental sequences using MEGAN4. Genome Res 21: 1552–1560.
Ivanova EP, Bakunina IY, Sawabe T, Hayashi K, Alexeeva YV, Zhukova NV et al (2002). Two species of culturable bacteria associated with degradation of brown algae Fucus evanescens. Microb Ecol 43: 242–249.
Lamy D, Obernosterer I, Laghdass M, Artigas F, Breton E, Grattepanche JD et al (2009). Temporal changes of major bacterial groups and bacterial heterotrophic activity during a Phaeocystis globosa bloom in the eastern English Channel. Aquat Microb Ecol 58: 95–107.
Liss PS, Malin G, Turner SM, Holligan PM . (1994). Dimethyl sulphide and Phaeocystis: a review. J Marine Syst 5: 41–53.
Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer K-H . (1996). Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 142: 1097–1106.
Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH . (1992). Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol 15: 593–600.
Mari X, Rassoulzadegan F, Brussaard CPDD, Wassmann P . (2005). Dynamics of transparent exopolymeric particles (TEP) production by Phaeocystis globosa under N- or P-limitation: a controlling factor of the retention/export balance. Harmful Algae 4: 895–914.
Middelboe M, Riemann L, Steward GF, Hansen V, Nybroe O . (2003). Virus-induced transfer of organic carbon between marine bacteria in a model community. Aquat Microb Ecol 33: 1–10.
Musat N, Halm H, Winterholler B, Hoppe P, Peduzzi S, Hillion F et al (2008). A single-cell view on the ecophysiology of anaerobic phototrophic bacteria. Proc Natl Acad Sci 105: 17861.
Olenina I, Hajdu S, Edler L, Andersson A . (2006). Biovolumes and size-classes of phytoplankton in the Baltic Sea. HELCOM Baltic Sea Environ Proc 106: 1–144.
Parada V, oacute nica, Herndl GJ, Weinbauer MG . (2006). Viral burst size of heterotrophic prokaryotes in aquatic systems. J Marine Biol Assoc UK 86: 613–621.
Pedros-Alio C . (2006). Marine microbial diversity: can it be determined? Trends Microbiol 14: 257–263.
Pernthaler A, Pernthaler J, Amann R . (2004). Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms. Mol Microb Ecol Manual 3: 711–726.
Pernthaler A, Pernthaler J, Eilers H, Amann R . (2001). Growth patterns of two marine isolates: Adaptations to substrate patchiness? Appl Environ Microbiol 67: 4077.
Polerecky L, Adam B, Milucka J, Musat N, Vagner T, Kuypers MMM . (2012). Look@NanoSIMS—a tool for the analysis of nanoSIMS data in environmental microbiology. Environ Microbiol 14: 1009–1023.
Proctor LM, Fuhrman JA . (1991). Roles of viral infection in organic particle flux. Marine Ecol Progress Series Oldendorf 69: 133–142.
Redfield AC . (1934). On the proportions of organic derivatives in sea water and their relation to the composition of plankton. James Johnstone Memorial Vol 176: 92.
Riemann L, Grossart HP . (2008). Elevated lytic phage production as a consequence of particle colonization by a marine Flavobacterium (Cellulophaga sp.). Microbial Ecol 56: 505–512.
Sandaa R-A, Gómez-Consarnau L, Pinhassi J, Riemann L, Malits A, Weinbauer MG et al (2009). Viral control of bacterial biodiversity – evidence from a nutrient-enriched marine mesocosm experiment. Environ Microbiol 11: 2585–2597.
Schäfer H . (2007). Isolation of Methylophaga spp. from marine dimethylsulfide-degrading enrichment cultures and identification of polypeptides induced during growth on dimethylsulfide. Appl Environ Microbiol 73: 2580–2591.
Shanks AL, Trent JD . (1979). Marine snow: Microscale nutrient patches. Limnol Oceanograph 24: 850–854.
Sheik AR, Brussaard CPD, Lavik G, Foster RA, Musat N, Adam B et al (2013). Viral infection of Phaeocystis globosa impedes release of chitinous star-like structures: quantification using single cell approaches. Environ Microbiol 15: 1441–1451.
Sun Y, Wolcott RD, Dowd SE . (2011). Tag-encoded FLX amplicon pyrosequencing for the elucidation of microbial and functional gene diversity in any environment. Methods Mol Biol (Clifton, Nj) 733: 129–141.
Suttle CA . (2005). Viruses in the sea. Nature 437: 356–361.
Teeling H, Fuchs BM, Becher D, Klockow C, Gardebrecht A, Bennke CM et al (2012). Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 336: 608–611.
Wilhelm SW, Suttle CA . (1999). Viruses and nutrient cycles in the sea. Bioscience 49: 781–788.
Zhou J, Bruns MA, Tiedje JM . (1996). DNA recovery from soils of diverse composition. Appl Environ Microbiol 62: 316–322.
Zubkov MV, Fuchs BM, Archer SD, Kiene RP, Amann R, Burkill PH . (2001). Linking the composition of bacterioplankton to rapid turnover of dissolved dimethylsulphoniopropionate in an algal bloom in the North Sea. Environ Microbiol 3: 304–311.
Acknowledgements
We thank Gabriele Klockgether, Daniela Franzke, Thomas Max, Birgit Adam, Anna Noordeloos and Evaline van Weerlee for their technical assistance. We are grateful to two anonymous reviewers for their valuable suggestions, which helped us to improve this manuscript. We thank the Max Planck Society (MPG) and Royal Netherlands Institute for Sea Research for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Sheik, A., Brussaard, C., Lavik, G. et al. Responses of the coastal bacterial community to viral infection of the algae Phaeocystis globosa. ISME J 8, 212–225 (2014). https://doi.org/10.1038/ismej.2013.135
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2013.135
Keywords
This article is cited by
-
Protist impacts on marine cyanovirocell metabolism
ISME Communications (2022)
-
Stress regulation of photosynthetic system of Phaeocystis globosa and their hemolytic activity
Journal of Oceanology and Limnology (2022)
-
Spatial and diel variations of the prokaryotic community in the Phaeocystis globosa blooms area of Beibu Gulf, China
Acta Oceanologica Sinica (2022)
-
Rapid microbial diversification of dissolved organic matter in oceanic surface waters leads to carbon sequestration
Scientific Reports (2020)
-
Autotrophic and heterotrophic acquisition of carbon and nitrogen by a mixotrophic chrysophyte established through stable isotope analysis
The ISME Journal (2017)