Abstract
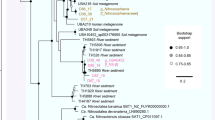
Novel lineages of the phylum Thaumarchaeota are endemic to thermal habitats, and may exhibit physiological capabilities that are not yet observed in members of this phylum. The primary goals of this study were to conduct detailed phylogenetic and functional analyses of metagenome sequence assemblies of two different thaumarchaeal populations found in high-temperature (65–72 °C), acidic (pH∼3) iron oxide and sulfur sediment environments of Yellowstone National Park (YNP). Metabolic reconstruction was coupled with detailed geochemical measurements of each geothermal habitat and reverse-transcriptase PCR to confirm the in situ activity of these populations. Phylogenetic analyses of ribosomal and housekeeping proteins place these archaea near the root of the thaumarchaeal branch. Metabolic reconstruction suggests that these populations are chemoorganotrophic and couple growth with the reduction of oxygen or nitrate in iron oxide habitats, or sulfur in hypoxic sulfur sediments. The iron oxide population has the potential for growth via the oxidation of sulfide to sulfate using a novel reverse sulfate reduction pathway. Possible carbon sources include aromatic compounds (for example, 4-hydroxyphenylacetate), complex carbohydrates (for example, starch), oligopeptides and amino acids. Both populations contain a type III ribulose bisphosphate carboxylase/oxygenase used for carbon dioxide fixation or adenosine monophosphate salvage. No evidence for the oxidation of ammonia was obtained from de novo sequence assemblies. Our results show that thermoacidophilic Thaumarchaeota from oxic iron mats and hypoxic sulfur sediments exhibit different respiratory machinery depending on the presence of oxygen versus sulfide, represent deeply rooted lineages within the phylum Thaumarchaeota and are endemic to numerous sites in YNP.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ackerman GG . (2006), Biogeochemical gradients and energetics in geothermal systems of Yellowstone National Park. MSc thesis, Montana State University: Bozeman, MT, USA, p 196.
APHA (1998a). Part 4500: S2− D. In: Clesceri LS, Greenberg AE, Eaton AD (eds) Standard Methods for the Examination of Water and Wastewater 20th edn. APHA: Washington, DC, USA, pp 4-165–166.
APHA (1998b). Part 4500: NH3 H. In: Clesceri LS, Greenberg AE, Eaton AD (eds) Standard Methods for the Examination of Water and Wastewater 20th edn. APHA: Washington, DC, USA, pp 4-111–112.
APHA (1998c). Part 4500: NO3− I. In: Clesceri LS, Greenberg AE, Eaton AD (eds) Standard Methods for the Examination of Water and Wastewater 20th edn. APHA: Washington, DC, USA, pp. 4-121–122.
Berg I, Kockelkorn D, Buckel W, Fuchs G . (2007). A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in archaea. Science 318: 1782–1786.
Bernstein HC, Beam JP, Kozubal MA, Carlson RP, Inskeep WP . (2013). In situ analysis of oxygen consumption and diffusive transport in high-temperature acidic iron-oxide microbial mats. Environ Microbiol 15: 2360–2370.
Boyd E, Jackson R, Encarnacion G, Zahn J, Beard T, Leavitt W et al (2007). Isolation, characterization, and ecology of sulfur-respiring Crenarchaea inhabiting acid-sulfate-chloride-containing geothermal springs in Yellowstone National Park. ApplEnviron Microbiol 73: 6669–6677.
Bridger S, Clarkson S, Stirrett K, DeBarry M, Lipscomb G, Schut G et al (2011). Deletion strains reveal metabolic roles for key elemental sulfur-responsive proteins in Pyrococcus furiosus. JBacteriol 193: 6498–6504.
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P . (2008a). Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6: 245–252.
Brochier-Armanet C, Forterre P, Gribaldo S . (2011b). Phylogeny and evolution of the Archaea: one hundred genomes later. Curr Opin Microbiol 14: 274–281.
Brochier-Armanet C, Gribaldo S, Forterre P . (2008b). A DNA topoisomerase IB in Thaumarchaeota testifies for the presence of this enzyme in the last common ancestor of Archaea and Eucarya. Biol Direct 3: 54.
Brochier-Armanet C, Gribaldo S, Forterre P . (2011a). Spotlight on the Thaumarchaeota. ISME J 6: 227–230.
de la Torre JR, Walker CB, Ingalls AE, Könneke M, Stahl DA . (2008). Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ Microbiol 10: 810–818.
D’ Imperio S, Lehr C, Breary M, McDermott T . (2007). Autecology of an arsenite chemolithotroph: sulfide constraints on function and distribution in a geothermal spring. Appl Environ Microbiol 73: 7067–7074.
Eddy S . (2011). Accelerated profile HMM searches. PLoS Comput Biol 7: e1002195.
Eme L, Reigstad LJ, Spang A, Lanzén A, Weinmaier T, Rattei T et al (2013). Metagenomics of Kamchatkan hot spring filaments reveal two new major (hyper)thermophilic lineages related to Thaumarchaeota. Res Microbiol 164: 425–438.
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB . (2005). Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102: 14683–14688.
Fuchs G, Boll M, Heider J . (2011). Microbial degradation of aromatic compounds—from one strategy to four. Nat Rev Microbiol 9: 803–816.
Gaudier M, Schuwirth BS, Westcott SL, Wigley DB . (2007). Structural basis of DNA replication origin recognition by an ORC protein. Science 317: 1213–1216.
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O . (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59: 307–321.
Hagen W, Silva P, Amorim M, Hagedoorn P, Wassink H, Haaker H et al (2000). Novel structure and redox chemistry of the prosthetic groups of the iron-sulfur flavoprotein sulfide dehydrogenase from Pyrococcus furiosus; evidence for a [2Fe-2S] cluster with Asp(Cys)(3) ligands. J Biol Inorg Chem 5: 527–534.
Hatzenpichler R . (2012). Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl Environ Microbiol 78: 7501–7510.
Hatzenpichler R, Lebedeva EV, Spieck E, Stoecker K, Richter A, Daims H et al (2008). A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc Natl Acad Sci USA 105: 2134–2139.
Huber H, Gallenberger M, Jahn U, Eylert E, Berg I, Kockelkorn D et al (2008). A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic archaeum Ignicoccus hospitalis. Proc Natl Acad Sci USA 105: 7851–7856.
Inskeep W, Ackerman G, Taylor W, Kozubal M, Korf S, Macur R . (2005). On the energetics of chemolithotrophy in nonequilibrium systems: case studies of geothermal springs in Yellowstone National Park. Geobiology 3: 297–317.
Inskeep W, Macur R, Harrison G, Bostick B, Fendorf S . (2004). Biomineralization of As(V)-hydrous ferric oxyhydroxide in microbial mats of an acid-sulfate-chloride geothermal spring, Yellowstone National Park. Geochim Cosmochim Acta 68: 3141–3155.
Inskeep W, Rusch D, Jay Z, Herrgard M, Kozubal M, Richardson T et al (2010). Metagenomes from high-temperature chemotrophic systems reveal geochemical controls on microbial community structure and function. PLoS One 5: e9773.
Inskeep WP, Jay ZJ, Herrgard MJ, Kozubal MA, Rusch DB, Tringe SG et al (2013). Phylogenetic and functional analysis of metagenome sequence from high-temperature archaeal habitats demonstrate linkages between metabolic potential and geochemistry. Front Microbiol 4: 95.
Inskeep WP, Jay ZJ, Tringe SG, Herrgard M, Rusch DB et al (2013). The YNP metagenome project: environmental parameters responsible for microbial distribution in the Yellowstone geothermal ecosystem. Front Microbiol 4: 67.
Jackson C, Langner H, Donahoe-Christiansen J, Inskeep W, McDermott T . (2001). Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ Microbiol 3: 532–542.
Joye S, Hollibaugh J . (1995). Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270: 623–625.
Junemann S . (1997). Cytochrome bd terminal oxidase. Biochim Biophys Acta Bioenerg 1321: 107–127.
Kirchman DL, Elifantz H, Dittel AI, Malmstrom RR, Cottrell MT . (2007). Standing stocks and activity of archaea and bacteria in the western arctic ocean. Limnol Oceanogr 52: 495–507.
Kozubal MA, Macur RE, Jay ZJ, Beam JP, Malfatti SA, Tringe SG et al (2012). Microbial iron cycling in acidic geothermal springs of Yellowstone National Park: integrating molecular surveys, geochemical processes, and isolation of novel Fe-active microorganisms. Front Microbiol 3: 109.
Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA . (2005). Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437: 543–546.
Langner H, Jackson C, McDermott T, Inskeep W . (2001). Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ SciTechnol 35: 3302–3309.
Le SQ, Gascuel O . (2008). An improved general amino acid replacement matrix. Mol Biol Evol 25: 1307–1320.
Lehtovirta-Morley L, Stoecker K, Vilcinskas A, Prosser J, Nicol G . (2011). Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA 108: 15892–15897.
Lloyd KG, Schreiber L, Peterson DG, Kjeldsen KU, Lever MA, Steen AD et al (2013). Predominant archaea in marine sediments degrade detrital proteins. Nature 496: 215–218.
Ma K, Adams M . (1994). Sulfide dehydrogenase from the hyperthermophilic archaon Pyrococcus furiosus—a new multifunctional enzyme involved in the reduction of elemental sulfur. J Bacteriol 176: 6509–6517.
Macur R, Langner H, Kocar B, Inskeep W . (2004). Linking geochemical processes with microbial community analysis: successional dynamics in an arsenic-rich, acid-sulphate-chloride geothermal spring. Geobiology 2: 163–177.
Makarova K, Yutin N, Bell S, Koonin E . (2010). Evolution of diverse cell division and vesicle formation systems in archaea. Nat Rev Microbiol 8: 731–741.
Markowitz V, Chen I-M, Palaniappan K, Chu K, Szeto E, Grechkin Y et al (2012). IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Res 40: D115–D122.
Martinez-Espinosa R, Dridge E, Bonete M, Butt J, Butler C, Sargent F et al (2007). Look on the positive side! The orientation, identification and bioenergetics of ‘archaeal’ membrane-bound nitrate reductases. FEMS Microbiol Lett 276: 129–139.
Miller P, Buschena D, Jones C, Holmes J . (2008). Transition from intensive tillage to no-tillage and organic diversified annual cropping systems. Agron J 100: 591–599.
Muβmann M, Brito I, Pitcher A, Damste J, Hatzenpichler R, Richter A et al (2011). Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proc Natl Acad Sci USA 108: 16771–16776.
Nunoura T, Takaki Y, Kakuta J, Nishi S, Sugahara J, Kazama H et al (2011). Insights into the evolution of archaea and eukaryotic protein modifier systems revealed by the genome of a novel archaeal group. Nucleic Acids Res 39: 3204–3223.
Ouverney CC, Fuhrman JA . (2000). Marine planktonic archaea take up amino acids. Appl Environ Microbiol 66: 4829–4833.
Pearson A, Huang Z, Ingalls A, Romanek C, Wiegel J, Freeman K et al (2004). Nonmarine crenarchaeol in Nevada hot springs. Appl Environ Microbiol 70: 5229–5237.
Pelve E, Lindas A, Martens-Habbena W, de la Torre J, Stahl D, Bernander R . (2011). Cdv-based cell division and cell cycle organization in the thaumarchaeon Nitrosopumilus maritimus. Mol Microbiol 82: 555–566.
Pester M, Schleper C, Wagner M . (2011). The Thaumarchaeota: an emerging view of their phylogeny and ecophysiology. Curr Opin Microbiol 14: 1–7.
Quatrini R, Appia-Ayme C, Denis Y, Jedlicki E, Holmes DS, Bonnefoy V . (2009). Extending the models for iron and sulfur oxidation in the extreme acidophile Acidithiobacillus ferrooxidans. BMC Genomics 10: 394.
Reigstad LJ, Richter A, Daims H, Urich T, Schwark L, Schleper C . (2008). Nitrification in terrestrial hot springs of Iceland and Kamchatka. FEMS Microbiol Ecol 64: 167–174.
Rinke C, Schwientek P, Sczyrba A, Ivanova N, Anderson I, Cheng J-F et al (2013). Insights into the phylogeny and coding potential of microbial dark matter. Nature 499: 431–437.
Samson R, Obita T, Freund S, Williams R, Bell S . (2008). A role for the ESCRT system in cell division in archaea. Science 322: 1710–1713.
Sato T, Atomi H, Imanaka T . (2007). Archaeal type III RuBisCOs function in a pathway for AMP metabolism. Science 315: 1003–1006.
Schleper C, Jurgens G, Jonuscheit M . (2005). Genomic studies of uncultivated archaea. Nat Rev Microbiol 3: 479–488.
Siebers B, Schonheit P . (2005). Unusual pathways and enzymes of central carbohydrate metabolism in archaea. Curr Opin Microbiol 8: 695–705.
Soderberg T . (2005). Biosynthesis of ribose-5-phophate and erythrose-4-phosphate in archaea: a phylogenetic analysis of archaeal genomes. Archaea 1: 347–352.
Spang A, Hatzenpichler R, Brochier-Armanet C, Rattei T, Tischler P, Spieck E et al (2010). Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends Microbiol 18: 331–340.
Spang A, Poehlein A, Offre P, Zumbrägel S, Haider S, Rychlik N et al (2012). The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis: insights into metabolic versatility and environmental adaptations. Environ Microbiol 14: 3122–3145.
Spear J, Barton H, Robertson C, Francis C, Pace N . (2007). Microbial community biofabrics in a geothermal mine adit. Appl Environ Microbiol 73: 6172–6180.
Stahl DA, de la Torre JR . (2012). Physiology and diversity of ammonia-oxidizing archaea. Annu Rev Microbiol 66: 83–101.
Suzuki I, Dular U, Kwok SC . (1974). Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J Bacteriol 120: 556–558.
Tabita F, Hanson T, Satagopan S, Witte B, Kreel N . (2008). Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Philos Trans R Soc B Biol Sci 363: 2629–2640.
Takacs-Vesbach C, Inskeep WP, Jay ZJ, Herrgard M, Rusch DB, Tringe SG et al (2013). Metagenome sequence analysis of filamentous microbial communities obtained from geochemically distinct geothermal channels reveals specialization of three aquificales lineages. Front Microbiol 4: 84.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S . (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739.
Teeling H, Meyerdierks A, Bauer M, Amann R, Glockner F . (2004). Application of tetranucleotide frequencies for the assignment of genomic fragments. Environ Microbiol 6: 938–947.
To T, Nordstrom D, Cunningham K, Ball J, McCleskey R . (1999). New method for the direct determination of dissolved Fe(III) concentration in acid mine waters. Environ Sci Technol 33: 807–813.
Tourna M, Stieglmeier M, Spang A, Könneke M, Schintlmeister A, Urich T et al (2011). Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA 108: 8420–8425.
Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ et al (2010). Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107: 8818–8823.
Walsh D, Zaikova E, Howes C, Song Y, Wright J, Tringe S et al (2009). Metagenome of a versatile chemolithoautotroph from expanding oceanic dead zones. Science 326: 578–582.
Weidler G, Dornmayr-Pfaffenhuemer M, Gerbl F, Heinen W, Stan-Lotter H . (2007). Communities of archaea and bacteria in a subsurface radioactive thermal spring in the Austrian Central Alps, and evidence of ammonia-oxidizing Crenarchaeota. Appl Environ Microbiol 73: 259–270.
Yutin N, Koonin EV . (2012). Archaeal origin of tubulin. Biol Direct 7: 10.
Zhang C, Ye Q, Huang Z, Li W, Chen J, Song Z et al (2008). Global occurrence of archaeal amoA genes in terrestrial hot springs. Appl Environ Microbiol 74: 6417–6426.
Acknowledgements
We appreciate support from the NSF-Integrative Graduate Education and Research Training Program to JPB and ZJJ (DGE 0654336), DOE-Pacific Northwest National Laboratory-Foundational Science Focus Area in Biological Interactions (Subcontract 112443), Douglas B Rusch at Indiana University, Bloomington, IN for NWF-PCA support, Susannah G Tringe at the DOE-JGI (Community Sequencing Program, CSP-787081) for metagenome sequencing performed at Dragon Spring (45 Mb Sanger) and Beowulf Spring (60 Mb 454), the J Craig Venter Institute for early Sanger sequencing at Beowulf Spring (12 Mb Sanger) and Justin O’Dea for positive amoA gene controls. The authors also appreciate comments from three anonymous reviewers that improved the quality of the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Beam, J., Jay, Z., Kozubal, M. et al. Niche specialization of novel Thaumarchaeota to oxic and hypoxic acidic geothermal springs of Yellowstone National Park. ISME J 8, 938–951 (2014). https://doi.org/10.1038/ismej.2013.193
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2013.193
Keywords
This article is cited by
-
Genome-resolved metagenomics inferred novel insights into the microbial community, metabolic pathways, and biomining potential of Malanjkhand acidic copper mine tailings
Environmental Science and Pollution Research (2023)
-
Diverse ecophysiological adaptations of subsurface Thaumarchaeota in floodplain sediments revealed through genome-resolved metagenomics
The ISME Journal (2022)
-
DDX1 from Cherry valley duck mediates signaling pathways and anti-NDRV activity
Veterinary Research (2021)
-
Evolution of the cytochrome bd oxygen reductase superfamily and the function of CydAA’ in Archaea
The ISME Journal (2021)
-
Metagenome-assembled genomes reveal unique metabolic adaptations of a basal marine Thaumarchaeota lineage
The ISME Journal (2020)