Abstract
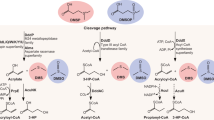
Diseases are an emerging threat to ocean ecosystems. Coral reefs, in particular, are experiencing a worldwide decline because of disease and bleaching, which have been exacerbated by rising seawater temperatures. Yet, the ecological mechanisms behind most coral diseases remain unidentified. Here, we demonstrate that a coral pathogen, Vibrio coralliilyticus, uses chemotaxis and chemokinesis to target the mucus of its coral host, Pocillopora damicornis. A primary driver of this response is the host metabolite dimethylsulfoniopropionate (DMSP), a key element in the global sulfur cycle and a potent foraging cue throughout the marine food web. Coral mucus is rich in DMSP, and we found that DMSP alone elicits chemotactic responses of comparable intensity to whole mucus. Furthermore, in heat-stressed coral fragments, DMSP concentrations increased fivefold and the pathogen’s chemotactic response was correspondingly enhanced. Intriguingly, despite being a rich source of carbon and sulfur, DMSP is not metabolized by the pathogen, suggesting that it is used purely as an infochemical for host location. These results reveal a new role for DMSP in coral disease, demonstrate the importance of chemical signaling and swimming behavior in the recruitment of pathogens to corals and highlight the impact of increased seawater temperatures on disease pathways.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmed T, Stocker R . (2008). Experimental verification of the behavioral foundation of bacterial transport parameters using microfluidics. Biophys J 95: 4481–4493.
Banin E, Israely T, Fine M, Loya Y, Rosenberg E . (2001). Role of endosymbiotic zooxanthellae and coral mucus in the adhesion of the coral-bleaching pathogen Vibrio shiloi to its host. FEMS Microbiol Lett 199: 33–37.
Barbara GM, Mitchell JG . (2003). Marine bacterial organisation around point-like sources of amino acids. FEMS Microbiol Ecol 43: 99–109.
Ben-Haim Y, Zicherman-Keren M, Rosenberg E . (2003). Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Environ Microbiol 69: 4236–4242.
Boin MA, Austin MJ, Hase CC . (2004). Chemotaxis in Vibrio cholerae. FEMS Microbiol Lett 239: 1–8.
Bourne DG, Garren M, Work TM, Rosenberg E, Smith GW, Harvell CD . (2009). Microbial disease and the coral holobiont. Trends Microbiol 17: 554–562.
Broadbent AD, Jones GB . (2004). DIMS and DMSP in mucus ropes, coral mucus, surface films and sediment pore waters from coral reefs in the Great Barrier Reef. Marine Freshwater Res 55: 849–855.
Brown BE, Bythell JC . (2005). Perspectives on mucus secretion in reef corals. Marine Ecol-Prog Ser 296: 291–309.
Brown DA, Berg HC . (1974). Temporal simulation of chemotaxis in Escherichia coli. Proc Natl Acad Sci USA 71: 1388–1392.
Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD et al. (2007). Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol 5: 1220–1227.
Croxen MA, Sisson G, Melano R, Hoffman PS . (2006). The Helicobacter pylori chemotaxis receptor TlpB (HP0103) is required for pH taxis and for colonization of the gastric mucosa. J Bacteriol 188: 2656–2665.
Debose JL, Nevitt GA . (2007). Investigating the association between pelagic fish and dimethylsulfoniopropionate in a natural coral reef system. Marine Freshwater Res 58: 720–724.
Fuse H, Takimura O, Murakami K, Yamaoka Y, Omori T . (2000). Utilization of dimethyl sulfide as a sulfur source with the aid of light by Marinobacterium sp. strain DMS-S1. Appl Environ Microbiol 66: 5527–5532.
Garcés E, Alacid E, Rene A, Petrou K, Simó R . (2013). Host-released dimethylsulphide activates the dinoflagellate parasitoid Parvilucifera sinerae. ISME J 7: 1065–1068.
Garren M, Azam F . (2012). Corals shed bacteria as a potential mechanism of resilience to organic matter enrichment. ISME J 6: 1159–1165.
Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E . (2009). Climate change and wildlife diseases: when does the host matter the most? Ecology 90: 912–920.
Jackson GA . (1987). Simulating chemosensory response of marine microorganisms. Limnol Oceanogr 32: 1253–1266.
Jatkar AA, Brown BE, Bythell JC, Guppy R, Morris NJ, Pearson JP . (2009). Measuring mucus thickness in reef corals using a technique devised for vertebrate applications. Marine Biol 2: 261–267.
Kiørboe T, Jackson GA . (2001). Marine snow, organic solute plumes, and optimal chemosensory behavior of bacteria. Limnol Oceanogr 46: 1309–1318.
Kirst G . (1989). Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol Plant Mol Biol 40: 21–53.
Kirst GO, Thiel C, Woll TH, Nothnagel J, Wanzek M, Ulmke R . (1991). Dimethylsulfoniopropionate (DMSP) in ice algae and its possible biological role. Marine Chem 35: 381–388.
Kühl M, Cohen Y, Dalsgaard T, Jørgensen BB, Revsbech NP . (1995). Microenvironment and photosynthesis of zooxanthellae in sceractinian corals studied with microsensors for O2, pH, and light. Marine Ecol-Prog Ser 117: 159–172.
Lesser MP, Weis VM, Patterson MR, Jokiel PL . (1994). Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis- diffusion barriers, inorganic carbon limiation, and biochemical plasticity. J Exp Marine Biol Ecol 178: 153–179.
Mass T, Genin A, Shavit U, Grinstein M, Tchernov D . (2010). Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proc Natl Acad Sci USA 107: 2527–2531.
Meron D, Efrony R, Johnson WR, Schaefer AL, Morris PJ, Rosenberg E et al. (2009). Role of flagella in virulence of the coral pathogen Vibrio coralliilyticus. Appl Environ Microbiol 75: 5704–5707.
Mydlarz LD, Jones LE, Harvell CD . (2006). Innate immunity environmental drivers and disease ecology of marine and freshwater invertebrates. Annu Rev Ecol Evol Syst 37: 251–288.
Pollock FJ, Wilson B, Johnson WR, Morris PJ, Willis BL, Bourne DG . (2010). Phylogeny of the coral pathogen Vibrio coralliilyticus. Environ Microbiol Rep 2: 172–178.
Raina JB, Tapiolas D, Willis BL, Bourne DG . (2009). Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol 75: 3492–3501.
Ritchie KB . (2006). Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Marine Ecol-Prog Ser 322: 1–14.
Schnitzer MJ . (1990). Strategies for chemotaxis. Symp Soc Gen Microbiol 46: 15–34.
Seymour JR, Ahmed T, Marcos, Stocker R . (2008). A microfluidic chemotaxis assay to study microbial behavior in diffusing nutrient patches. Limnol Oceanogr-Meth 6: 477–488.
Seymour JR, Simó R, Ahmed T, Stocker R . (2010). Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science 329: 342–345.
Simó R, Vila-Costa M, Alonso-Sáez L, Cardelús C, Guadayol O et al. (2009). Annual DMSP contribution to S and C fluxes through phytoplankton and bacterioplankton in a NS Mediterranean coastal site. Aquat Microb Ecol 57: 43–55.
Spagnolie S, Liu B, Powers TR . (2013). Locomotion of helical bodies in viscoelastic fluids: Enhanced swimming at large helical amplitudes. Phys Rev Lett 111: 068101.
Stefels J . (2000). Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J Sea Res 43: 183–197.
Stocker R, Seymour JR, Samadani A, Hunt DE, Polz MF . (2008). Rapid chemotactic response enables marine bacteria to exploit ephemeral microscale nutrient patches. Proc Natl Acad Sci USA 105: 4209–4214.
Sunda W, Kieber DJ, Kiene RP, Huntsman S . (2002). An antioxidant function for DMSP and DMS in marine algae. Nature 418: 317–320.
Sussman M, Mieog JC, Doyle J, Victor S, Willis BL, Bourne DG . (2009). Vibrio zinc-metalloprotease causes photoinactivation of coral endosymbionts and coral tissue lesions. PLoS One 4: e4511.
Sussman M, Willis BL, Victor S, Bourne DG . (2008). Coral pathogens identified for White Syndrome (WS) epizootics in the Indo-Pacific. PLoS One 3: e2393.
Tapiolas DM, Raina JB, Lutz A, Willis BL, Motti CA . (2013). Direct measurement of dimethysulfoniopropionate (DMSP) in reef-building corals using quantitative nuclear magnetic resonance (qNMR) spectroscopy. J Exp Marine Biol Ecol 443: 85–89.
Taylor JR, Stocker R . (2012). Trade-offs of chemotactic foraging in turbulent water. Science 338: 675–679.
Van Alstyne KL, Schupp P, Slattery M . (2006). The distribution of dimethylsulfoniopropionate in tropical Pacific coral reef invertebrates. Coral Reefs 25: 321–327.
Verbrugghe E, Boyen F, Gaastra W, Bekhuis L, Leyman B, Van Parys A et al. (2012). The complex interplay between stress and bacterial infections in animals. Vet Microbiol 155: 115–127.
Wahl M, Kroger K, Lenz M . (1998). Non-toxic protection against epibiosis. Biofouling 12: 205–226.
Zubkov MV, Fuchs BM, Archer SD, Kiene RP, Amann R, Burkill PH . (2001). Linking the composition of bacterioplankton to rapid turnover of dissolved dimethylsulphoniopropionate in an algal bloom in the North Sea. Environ Microbiol 3: 304–311.
Acknowledgements
We thank JS Guasto, C Motti, F Nosratpour, PJ Ralph, T Santiano-McHatton and the Birch Aquarium at Scripps for assistance. Credit for Figure 4: G Gorick, M Garren and R Stocker. This work was supported by the Human Frontiers in Science Program award no. RGY0089 to RS and JRS to a Gordon and Betty Moore Foundation Investigator Grant to RS, by the Australian Research Council Grant DP110103091 to JRS, by a Samsung Scholarship to KS, by funds through the marine microbiology program at AIMS to DGB, by the post-graduate award from the Department of Environmental Science and Climate Change Cluster at the University of Technology Sydney and the Australian Coral Reef Society Terry Walker award 2012 to JT and by NSF awards OCE-0744641-CAREER, CBET-1066566 and CBET-0966000 to RS. We are grateful to the Great Barrier Reef Marine Park Authority for coral collection permits G09/31733.1 (PJ Ralph, University of Technology Sydney) and G12/35236.1 (Australian Institute of Marine Science).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Garren, M., Son, K., Raina, JB. et al. A bacterial pathogen uses dimethylsulfoniopropionate as a cue to target heat-stressed corals. ISME J 8, 999–1007 (2014). https://doi.org/10.1038/ismej.2013.210
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2013.210
Keywords
This article is cited by
-
Strong chemotaxis by marine bacteria towards polysaccharides is enhanced by the abundant organosulfur compound DMSP
Nature Communications (2023)
-
Global Distribution of Hard Coral Pathogen Vibrio coralliilyticus; an Ensemble Modelling Approach
Thalassas: An International Journal of Marine Sciences (2023)
-
Effects of elevated temperature and acidification on sulfate assimilation and reduction of microalgae
Journal of Applied Phycology (2023)
-
A meta-analysis of the stony coral tissue loss disease microbiome finds key bacteria in unaffected and lesion tissue in diseased colonies
ISME Communications (2023)
-
The ecological roles of bacterial chemotaxis
Nature Reviews Microbiology (2022)