Abstract
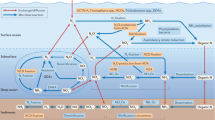
Bacteria of the marine Roseobacter clade are characterised by their ability to utilise a wide range of organic and inorganic compounds to support growth. Trimethylamine (TMA) and trimethylamine N-oxide (TMAO) are methylated amines (MA) and form part of the dissolved organic nitrogen pool, the second largest source of nitrogen after N2 gas, in the oceans. We investigated if the marine heterotrophic bacterium, Ruegeria pomeroyi DSS-3, could utilise TMA and TMAO as a supplementary energy source and whether this trait had any beneficial effect on growth. In R. pomeroyi, catabolism of TMA and TMAO resulted in the production of intracellular ATP which in turn helped to enhance growth rate and growth yield as well as enhancing cell survival during prolonged energy starvation. Furthermore, the simultaneous use of two different exogenous energy sources led to a greater enhancement of chemoorganoheterotrophic growth. The use of TMA and TMAO primarily as an energy source resulted in the remineralisation of nitrogen in the form of ammonium, which could cross feed into another bacterium. This study provides greater insight into the microbial metabolism of MAs in the marine environment and how it may affect both nutrient flow within marine surface waters and the flux of these climatically important compounds into the atmosphere.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Arata H, Shimizu M, Takamiya K . (1992). Purification and properties of trimethylamine N-oxide reductase from aerobic photosynthetic bacterium Roseobacter denitrificans. J Biochem 112: 470–475.
Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F . (1983). The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10: 257–263.
Boden R, Cunliffe M, Scanlan J, Moussard H, Kits KD, Klotz MG et al. (2011a). Complete genome sequence of the aerobic marine methanotroph Methylomonas methanica MC09. J Bacteriol 193: 7001–7002.
Boden R, Murrell JC, Schäfer H . (2011b). Dimethylsulfide is an energy source for the heterotrophic marine bacterium Sagittula stellata. FEMS Microbiol Lett 322: 188–193.
Buchan A, González JM, Moran MA . (2005). Overview of the Marine Roseobacter Lineage. Appl Environ Microbiol 71: 5665–5677.
Capone DG, Bronk DA, Mulholland MR, Carpenter EJ . (2008) Nitrgoen in the marine environment 2nd edn. Elsevier: Amsterdam, Netherlands.
Carpenter LJ, Archer SD, Beale R . (2012). Ocean-atmosphere trace gas exchange. Chem Soc Rev 41: 6473–6506.
Chen Y . (2012). Comparative genomics of methylated amine utilization by marine Roseobacter clade bacteria and development of functional gene markers (tmm, gmaS). Environ Microbiol 14: 2308–2322.
Chen Y, Patel NA, Crombie A, Scrivens JH, Murrell JC . (2011). Bacterial flavin-containing monooxygenase is trimethylamine monooxygenase. Proc Natl Acad Sci USA 108: 17791–17796.
Chistoserdova L . (2011). Modularity of methylotrophy, revisited. Environ Microbiol 13: 2603–2622.
Chistoserdova L, Kalyuzhnaya MG, Lidstrom ME . (2009). The expanding world of methylotrophic metabolism. Annu Rev Microbio l63: 477–499.
Cunliffe M . (2012). Physiological and metabolic effects of carbon monoxide oxidation in the model marine bacterioplankton Ruegeria pomeroyi DSS-3. Appl Environ Microbiol 79: 738–740.
Eiler A . (2006). Evidence for the ubiquity of mixotrophic bacteria in the upper ocean: implications and consequences. Appl Environ Microbiol 72: 7431–7437.
Eppley RW, Peterson BJ . (1979). Particulate organic matter flux and planktonic new production in the deep ocean. Nature 282: 677–680.
Falkowski PG, Barber RT, Smetacek V . (1998). Biogeochemical controls and feedbacks on ocean primary production. Science 281: 200–206.
Fitzsimons MF, Kahni-danon B, Dawitt M . (2001). Distributions and adsorption of the methylamines in the inter-tidal sediments of an East Anglian Estuary. Environ Exp Bot 46: 225–236.
Garber JH . (1984). Laboratory study of nitrogen and phosphorus remineralization during the decomposition of coastal plankton and seston. Est Coast Shelf Sci 18: 685–702.
Gibb SW, Hatton AD . (2004). The occurrence and distribution of trimethylamine-N-oxide in Antarctic coastal waters. Mar Chem 91: 65–75.
Gibb SW, Mantoura RFC, Liss PS, Barlow RG . (1999). Distributions and biogeochemistries of methylamines and ammonium in the Arabian Sea. Deep-Sea Res PT II 46: 593–615.
Gifford SM, Sharma S, Booth M, Moran MA . (2013). Expression patterns reveal niche diversification in a marine microbial assemblage. ISME J 7: 281–298.
González JM, Covert JS, Whitman WB, Henriksen JR, Mayer F, Scharf B et al. (2003). Silicibacter pomeroyi sp. nov. and Roseovarius nubinhibens sp. nov., dimethylsulfoniopropionate-demethylating bacteria from marine environments. Int J Syst Evol Micr 53: 1261–1269.
González JM, Simó R, Massana R, Covert JS, Casamayor EO, Pedrós-Alió C et al. (2000). Bacterial community structure associated with a dimethylsulfoniopropionate-producing north atlantic algal bloom. Appl Environ Microbiol 66: 4237–4246.
Green DH, Shenoy DM, Hart MC, Hatton AD . (2011). Coupling of dimethylsulfide oxidation to biomass production by a marine Flavobacterium. Appl Environ Microbiol 77: 3137–3140.
Gómez-Consarnau L, Akram N, Lindell K, Pedersen A, Neutze R, Milton DL et al. (2010). proteorhodopsin phototrophy promotes survival of marine bacteria during starvation. PLoS Biol 8: e1000358.
Hahnke S, Brock NL, Zell C, Simon M, Dickschat JS, Brinkhoff T . (2013). Physiological diversity of Roseobacter clade bacteria co-occurring during a phytoplankton bloom in the North Sea. Syst Appl Microbiol 36: 39–48.
Halsey KH, Carter AE, Giovannoni SJ . (2012). Synergistic metabolism of a broad range of C1 compounds in the marine methylotrophic bacterium HTCC2181. Environ Microbiol 14: 630–640.
King GM . (1984). Metabolism of trimethylamine, choline, and glycine betaine by sulfate-reducing and methanogenic bacteria in marine sediments. Appl Environ Microbiol 48: 719–725.
Lidbury I, Murrell JC, Chen Y . (2014). Trimethylamine N-oxide metabolism by abundant marine heterotrophic bacteria. Proc Natl Acad Sci USA 111: 2710–2715.
Moran MA, Buchan A, Gonzalez JM, Heidelberg JF, Whitman WB, Kiene RP et al. (2004). Genome sequence of Silicibacter pomeroyi reveals adaptations to the marine environment. Nature 432: 910–913.
Moran MA, Miller WL . (2007). Resourceful heterotrophs make the most of light in the coastal ocean. Nat Rev Micro 5: 792–800.
Nelson CE, Carlson CA, Ewart CS, Halewood ER . (2014). Community differentiation and population enrichment of Sargasso Sea bacterioplankton in the euphotic zone of a mesoscale mode-water eddy. Environ Microbiol 16: 871–887.
Newton RJ, Griffin LE, Bowles KM, Meile C, Gifford S, Givens CE et al. (2010). Genome characteristics of a generalist marine bacterial lineage. ISME J 4: 784–798.
Quinn PK, Charlson RJ, Bates TS . (1988). Simultaneous observations of ammonia in the atmosphere and ocean. Nature 335: 336–338.
Schäfer H . (2007). Isolation of Methylophaga spp. from marine dimethylsulfide-degrading enrichment cultures and identification of polypeptides induced during growth on dimethylsulfide. Appl Environ Microbiol 73: 2580–2591.
Smith DP, Thrash JC, Nicora CD, Lipton MS, Burnum-Johnson KE, Carini P et al. (2013). Proteomic and transcriptomic analyses of “Candidatus Pelagibacter ubique” describe the first pii-independent response to nitrogen limitation in a free-living Alphaproteobacterium. MBio 4: e00133–12.
Sorokin DY, Tourova TP, Muyzer G . (2005). Citreicella thiooxidans gen. nov., sp. nov., a novel lithoheterotrophic sulfur-oxidizing bacterium from the Black Sea. Syst and Appl Microbiol 28: 679–687.
Sowell SM, Abraham PE, Shah M, Verberkmoes NC, Smith DP, Barofsky DF et al. (2011). Environmental proteomics of microbial plankton in a highly productive coastal upwelling system. ISME J 5: 856–865.
Sowell SM, Wilhelm LJ, Norbeck AD, Lipton MS, Nicora CD, Barofsky DF et al. (2008). Transport functions dominate the SAR11 metaproteome at low-nutrient extremes in the Sargasso Sea. ISME J 3: 93–105.
Steindler L, Schwalbach MS, Smith DP, Chan F, Giovannoni SJ . (2011). Energy starved Candidatus Pelagibacter Ubique substitutes light-mediated ATP production for endogenous carbon respiration. PLoS One 6: e19725.
Sun J, Steindler L, Thrash JC, Halsey KH, Smith DP, Carter AE et al. (2011). One carbon metabolism in SAR11 pelagic marine bacteria. PLoS One 6: e23973.
Todd JD, Kirkwood M, Newton-Payne S, Johnston AWB . (2012). DddW, a third DMSP lyase in a model Roseobacter marine bacterium, Ruegeria pomeroyi DSS-3. ISME J 6: 223–226.
Treberg JR, Speers-Roesch B, Piermarini PM, Ip YK, Ballantyne JS, Driedzic WR . (2006). The accumulation of methylamine counteracting solutes in elasmobranchs with differing levels of urea: a comparison of marine and freshwater species. J Exp Biol 209: 860–870.
Wagner-Dobler I, Ballhausen B, Berger M, Brinkhoff T, Buchholz I, Bunk B et al. (2009). The complete genome sequence of the algal symbiont Dinoroseobacter shibae: a hitchhiker’s guide to life in the sea. ISME J 4: 61–77.
Williams TJ, Long E, Evans F, DeMaere MZ, Lauro FM, Raftery MJ et al. (2012). A metaproteomic assessment of winter and summer bacterioplankton from Antarctic Peninsula coastal surface waters. ISME J 6: 1883–1900.
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN . (1982). Living with water stress: evolution of osmolyte systems. Science 217: 1214–1222.
Zehr JP, Kudela RM . (2011). Nitrogen cycle of the open ocean: From genes to Ecosystems. Annu Rev Mar Sci 4: 197–225.
Zhu Y, Jameson E, Crosatti M, Schäfer H, Rajakumar K, Bugg TDH et al. (2014). Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci USA 111: 4268–4273.
Acknowledgements
We thank Dr Rich Boden, Plymouth University, UK, for his advice on chemostat cultures. This work was supported by the Natural and Environment Research Council (NERC) through a research studentship (to IDEAL) and a NERC fellowship (NE/H016236/1; to YC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Lidbury, I., Murrell, J. & Chen, Y. Trimethylamine and trimethylamine N-oxide are supplementary energy sources for a marine heterotrophic bacterium: implications for marine carbon and nitrogen cycling. ISME J 9, 760–769 (2015). https://doi.org/10.1038/ismej.2014.149
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2014.149
This article is cited by
-
The foul play of two dietary metabolites trimethylamine (TMA) and trimethylamine N-oxide (TMAO) on human health and the role of microbes in mitigating their effects
Nutrire (2023)
-
Transporter characterisation reveals aminoethylphosphonate mineralisation as a key step in the marine phosphorus redox cycle
Nature Communications (2021)
-
Distinct influence of trimethylamine N-oxide and high hydrostatic pressure on community structure and culturable deep-sea bacteria
Journal of Oceanology and Limnology (2020)
-
Effects of water recirculation rate on the microbial community and water quality in relation to the growth and survival of white shrimp (Litopenaeus vannamei)
BMC Microbiology (2019)
-
A new family of uncultivated bacteria involved in methanogenesis from the ubiquitous osmolyte glycine betaine in coastal saltmarsh sediments
Microbiome (2019)