Abstract
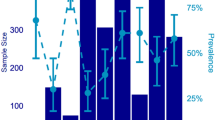
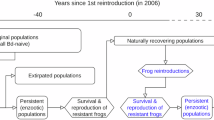
The fungal pathogen Batrachochytrium dendrobatidis (Bd) has caused declines and extinctions in amphibians worldwide, and there is increasing evidence that some strains of this pathogen are more virulent than others. While a number of putative virulence factors have been identified, few studies link these factors to specific epizootic events. We documented a dramatic decline in juvenile frogs in a Bd-infected population of Cascades frogs (Rana cascadae) in the mountains of northern California and used a laboratory experiment to show that Bd isolated in the midst of this decline induced higher mortality than Bd isolated from a more stable population of the same species of frog. This highly virulent Bd isolate was more toxic to immune cells and attained higher density in liquid culture than comparable isolates. Genomic analyses revealed that this isolate is nested within the global panzootic lineage and exhibited unusual genomic patterns, including increased copy numbers of many chromosomal segments. This study integrates data from multiple sources to suggest specific phenotypic and genomic characteristics of the pathogen that may be linked to disease-related declines.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Adams MJ, Chelgren ND, Reinitz D, Cole RA, Rachowicz LJ, Galvan S et al. (2010). Using occupancy models to understand the distribution of an amphibian pathogen Batrachochytrium dendrobatidis. Ecol Appl 20: 289–302.
Bates D, Maechler M, Bolker B . (2014). lme4: Linear mixed-effects models using Eigen and S4. Available from http://cran.r-project.org/web/packages/lme4/index.html.
Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL et al. (1998). Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95: 9031–9036.
Berger L, Marantelli G, Skerratt LL, Speare R . (2005). Virulence of the amphibian chytrid fungus Batrachochytrium dendrobatidis varies with the strain. Dis Aquat Organ 68: 47–50.
Boyle DG, Boyle DB, Olsen V, Morgan JA, Hyatt AD . (2004). Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ 60: 141–148.
Briggs CJ, Knapp RA, Vredenburg VT . (2010). Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci USA 107: 9695–9700.
Bull JJ . (1994). Perspective: virulence. Evolution 48: 1423–1437.
Crump ML, Scott NJJ . (1994). Standard techniques for inventory and monitoring: visual encounter surveys. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS, (eds) Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians. Smithsonian Institution: Washington, D.C., USA, pp 84–92.
Ebert D, Bull JJ . (2008). The evolution and expression of virulence. In: Stearns SC, Koella JC, (eds) Evolution in Health and Disease 2nd edn Oxford. University Press: New York, NY, USA, pp 154–167.
Ewald PW . (1994) Evolution of Infectious Disease. Oxford University Press: New York, NY, USA.
Farrer RA, Weinert LA, Bielby J, Garner TJ, Balloux F, Clare F et al. (2011). Multiple emergences of genetically diverse amphibian-infecting chytrids include a gobalized hypervirulent recombinant lineage. Proc Natl Acad Sci USA 108: 18732–18736.
Farrer RA, Henk DA, Garner TWJ, Balloux F, Woodhams DC, Fisher MC . (2013). Chromosomal copy number variation, selection and uneven rates of recombination reveal cryptic genome diversity linked to pathogenicity. PLoS Genet 9: e1003703.
Fellers GM, Pope KL, Stead JE, Koo MS, Welsh HH . (2008). Turning population trend monitoring into active conservation: can we save the Cascades frog (Rana Cascadae) in the Lassen region of California? Herpetol Conserv Bio 3: 28–39.
Fisher MC, Bosch J, Yin Z, Stead DA, Walker J, Selway L et al. (2009a). Proteomic and phenotypic profiling of the amphibian pathogen Batrachochytrium dendrobatidis shows that genotype is linked to virulence. Mol Ecol 18: 415–429.
Fisher MC, Garner TWJ, Walker SF . (2009b). Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol 63: 291–310.
Fites JS, Ramsey JP, Holden WM, Collier SP, Sutherland DM, Reinert LK et al. (2013). The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342: 366–369.
Gahl MK, Longcore JE, Houlahan JE . (2012). Varying responses of northeastern North American amphibians to the chytrid pathogen Batrachochytrium dendrobatidis. Conserv Biol 26: 135–141.
Garcia TS, Romansic JM, Blaustein AR . (2006). Survival of three species of anuran metamorphs exposed to UV-B radiation and the pathogenic fungus Batrachochytrium dendrobatidis. Dis Aquat Organ 72: 163–169.
Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D et al. (2007). Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Organ 73: 175–192.
Joneson S, Stajich JE, Shiu SH, Rosenblum EB . (2011). Genomic transition to pathogenicity in chytrid fungi. PLoS Pathogens 7: e1002338.
Kilpatrick AM, Briggs CJ, Daszak P . (2010). The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol Evol 25: 109–118.
Kriger KM, Pereoglou F, Hero JM . (2007). Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conserv Biol 21: 1280–1290.
LaDeau SL, Kilpatrick AM, Marra PP . (2007). West Nile virus emergence and large-scale declines of North American bird populations. Nature 447: 710–U713.
Langhammer PF, Lips KR, Burrowes PA, Tunstall T, Palmer CM, Collins JP . (2013). A fungal pathogen of amphibians, Batrachochytrium dendrobatidis, attenuates in pathogenicity with in vitro passages. PLoS One 8: e77630.
Lenski RE, May RM . (1994). The evolution of virulence in parasites and pathogens: Reconciliation between two competing hypotheses. J Theor Biol 169: 253–165.
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J et al. (2006). Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA 103: 3165–3170.
Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L et al. (2003). Transmission dynamics and control of severe acute respiratory syndrome. Science 300: 1966–1970.
Longcore JE, Pessier AP, Nichols DK . (1999). Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mycologia 91: 219–227.
Pearl CA, Adams MJ, Bury RB, Wente WH, McCreary B . (2009). Evaluating amphibian declines with site revisits and occupancy models: status of montane anurans in the Pacific northwest USA. Diversity 1: 166–181.
Phillips BL, Puschendorf R . (2013). Do pathogens become more virulent as they spread? Evidence from the amphibian declines in Central America. Proc R Soc B Biol Sci 280: 20131290.
Piovia-Scott J, Pope KL, Lawler SP, Cole EM, Foley JE . (2011). Factors related to the distribution and prevalence of the fungal pathogen Batrachochytrium dendrobatidis in Rana cascadae and other amphibians in the Klamath Mountains. Biol Conserv 144: 2913–2921.
Pope KL . (2008). Assessing changes in amphibian population dynamics following experimental manipulations of introduced fish. Conserv Biol 22: 1572–1581.
Pope KL, Brown C, Hayes M, Green G, Macfarlane D . (2014) Cascades Frog Conservation Assessment. Pacific Southwest Research Station, United States Forest Service: Albany, CA, USA.
R Development Core Team. (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria.
Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR . (2013). Disease and thermal acclimation in a more variable and unpredictable climate. Nat Clim Change 3: 146–151.
Retallick RW, Miera V, Richards KL, Field KJ, Collins JP . (2006). A non-lethal technique for detecting the chytrid fungus Batrachochytrium dendrobatidis on tadpoles. Dis Aquat Organ 72: 77–85.
Retallick RW, Miera V . (2007). Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis Aquat Organ 75: 201–207.
Richards-Zawacki CL . (2010). Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc R Soc B 277: 519–528.
Rodriguez D, Becker CG, Pupin NC, Haddad CF, Zamudio KR . (2014). Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic Forest of Brazil. Mol Ecol 23: 774–787.
Rosenblum EB, Poorten TJ, Joneson S, Settles M . (2012). Substrate-specific gene expression in Batrachochytrium dendrobatidis, the chytrid pathogen of amphibians. PLoS ONE 7: e49924.
Rosenblum EB, James TY, Zamudio KR, Poorten TJ, Ilut D, Rodriguez D et al. (2013). Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc Natl Acad Sci USA 110: 9385–9390.
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD et al. (2007). Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4: 125–134.
Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG et al. (2009). Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459: 1122–U1107.
Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ . (2010). Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA 107: 9689–9694.
Wake DB, Vredenburg VT . (2008). Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA 105: 11466–11473.
Yang ZH . (2007). PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24: 1586–1591.
Zolan ME, Pukkila PJ . (1986). Inheritance of DNA methylation in coprinus-cinereus. Mol Cell Biol 6: 195–200.
Acknowledgements
Monty Larson, Kevin Aceituno and Cathy Johnson and many others helped in field data collection; Carlos Davidson and Daniella Reagan helped with the amphibian experiment. This research was supported by grants from the California Department of Fish and Wildlife, the United States Fish and Wildlife Service, Lassen National Forest and the National Science Foundation (IOS-1121758 and IOS-12244804).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Piovia-Scott, J., Pope, K., Joy Worth, S. et al. Correlates of virulence in a frog-killing fungal pathogen: evidence from a California amphibian decline. ISME J 9, 1570–1578 (2015). https://doi.org/10.1038/ismej.2014.241
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2014.241
This article is cited by
-
Amphibian chytridiomycosis outbreak dynamics are linked with host skin bacterial community structure
Nature Communications (2018)
-
Host–pathogen dynamics among the invasive American bullfrog (Lithobates catesbeianus) and chytrid fungus (Batrachochytrium dendrobatidis)
Hydrobiologia (2018)
-
Batrachochytrium dendrobatidis and the Decline and Survival of the Relict Leopard Frog
EcoHealth (2017)
-
Recent Emergence of a Chytrid Fungal Pathogen in California Cascades Frogs (Rana cascadae)
EcoHealth (2017)
-
Greater Species Richness of Bacterial Skin Symbionts Better Suppresses the Amphibian Fungal Pathogen Batrachochytrium Dendrobatidis
Microbial Ecology (2017)