Abstract
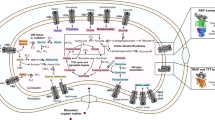
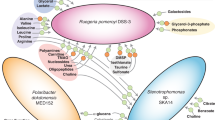
Vitamin traffic, the production of organic growth factors by some microbial community members and their use by other taxa, is being scrutinized as a potential explanation for the variation and highly connected behavior observed in ocean plankton by community network analysis. Thiamin (vitamin B1), a cofactor in many essential biochemical reactions that modify carbon–carbon bonds of organic compounds, is distributed in complex patterns at subpicomolar concentrations in the marine surface layer (0–300 m). Sequenced genomes from organisms belonging to the abundant and ubiquitous SAR11 clade of marine chemoheterotrophic bacteria contain genes coding for a complete thiamin biosynthetic pathway, except for thiC, encoding the 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP) synthase, which is required for de novo synthesis of thiamin’s pyrimidine moiety. Here we demonstrate that the SAR11 isolate ‘Candidatus Pelagibacter ubique’, strain HTCC1062, is auxotrophic for the thiamin precursor HMP, and cannot use exogenous thiamin for growth. In culture, strain HTCC1062 required 0.7 zeptomoles per cell (ca. 400 HMP molecules per cell). Measurements of dissolved HMP in the Sargasso Sea surface layer showed that HMP ranged from undetectable (detection limit: 2.4 pM) to 35.7 pM, with maximum concentrations coincident with the deep chlorophyll maximum. In culture, some marine cyanobacteria, microalgae and bacteria exuded HMP, and in the Western Sargasso Sea, HMP profiles changed between the morning and evening, suggesting a dynamic biological flux from producers to consumers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abascal F, Zardoya R, Posada D . (2005). ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21: 2104–2105.
Barada LP, Cutter L, Montoya JP, Webb EA, Capone DG, Sañudo-Wilhelmy SA . (2013). The distribution of thiamin and pyridoxine in the western tropical North Atlantic Amazon River plume. Front Microbiol 4: 25.
Bertrand EM, Allen AE . (2012). Influence of vitamin B auxotrophy on nitrogen metabolism in eukaryotic phytoplankton. Front Microbiol 3: 375.
Bertrand EM, Allen AE, Dupont CL, Norden-Krichmar TM, Bai J, Valas RE et al. (2012). Influence of cobalamin scarcity on diatom molecular physiology and identification of a cobalamin acquisition protein. Proc Natl Acad Sci USA 109: E1762–E1771.
Bertrand EM, Saito MA, Rose JM, Riesselman CR, Lohan MC, Noble AE et al. (2007). Vitamin B12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnol Oceanogr 52: 1079–1093.
Brown MR, Mular M, Miller I, Farmer C, Trenerry C . (1999). The vitamin content of microalgae used in aquaculture. J Appl Phycol 11: 247–255.
Button D . (1968). Selective thiamine removal from culture media by ultraviolet irradiation. Appl Microbiol 16: 530–531.
Carini P, Steindler L, Beszteri S, Giovannoni SJ . (2013). Nutrient requirements for growth of the extreme oligotroph ‘Candidatus Pelagibacter ubique’ HTCC1062 on a defined medium. ISME J 7: 592–602.
Carlson CA, Morris R, Parsons R, Treusch AH, Giovannoni SJ, Vergin K . (2009). Seasonal dynamics of SAR11 populations in the euphotic and mesopelagic zones of the northwestern Sargasso Sea. ISME J 3: 283–295.
Carlucci A, Bowes PM . (1970a). Production of vitamin B12, thiamine, and biotin by phytoplankton. J Phycol 6: 351–357.
Carlucci A, Bowes PM . (1972). Vitamin B12, thiamine, and biotin contents of marine phytoplankton. J Phycol 8: 133–137.
Carlucci A, Bowes PM . (1970b). Vitamin production and utilization by phytoplankton in mixed culture. J Phycol 6: 393–400.
Castresana J . (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17: 540–552.
Croft M, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG . (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438: 90–93.
Croft M, Warren M, Smith A . (2006). Algae need their vitamins. Eukaryotic Cell 5: 1175–1183.
Croft MT, Moulin M, Webb ME, Smith AG . (2007). Thiamine biosynthesis in algae is regulated by riboswitches. Proc Natl Acad Sci USA 104: 20770–20775.
Downs DM . (1992). Evidence for a new, oxygen-regulated biosynthetic pathway for the pyrimidine moiety of thiamine in Salmonella typhimurium. J Bacteriol 174: 1515–1521.
Droop MR . (1958). Requirement for thiamine among some marine and supra-littoral protista. Mar Biol Assoc UK 37: 323–329.
Dupont CL, Rusch DB, Yooseph S, Lombardo M-J, Richter RA, Valas R et al. (2012). Genomic insights to SAR86, an abundant and uncultivated marine bacterial lineage. ISME J 6: 1186–1199.
Edgar RC . (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797.
Fuhrman JA, Hewson I, Schwalbach MS, Steele JA, Brown MV, Naeem S . (2006). Annually reoccurring bacterial communities are predictable from ocean conditions. Proc Natl Acad Sci USA 103: 13104–13109.
Giovannoni SJ, Hayakawa DH, Tripp HJ, Stingl U, Givan SA, Cho J-C et al. (2008). The small genome of an abundant coastal ocean methylotroph. Environ Microbiol 10: 1771–1782.
Giovannoni SJ, Tripp HJ, Givan S, Podar M, Vergin KL, Baptista D et al. (2005). Genome streamlining in a cosmopolitan oceanic bacterium. Science (New York, NY) 309: 1242–1245.
Gold K . (1968). Some factors affecting the stability of thiamine. Limnol Oceanogr 13: 185–188.
Gold K, Roels OA, Bank H . (1966). Temperature dependent destruction of thiamine in seawater. Limnol Oceanogr 11: 410–413.
Helliwell KE, Wheeler GL, Smith AG . (2013). Widespread decay of vitamin-related pathways: coincidence or consequence? Trends Genet 29: 469–478.
Ishida S, Tazuya-Murayama K, Kijima Y, Yamada K . (2008). The direct precursor of the pyrimidine moiety of thiamin is not urocanic acid but histidine in Saccharomyces cerevisiae. J Nutr Sci Vitaminol (Tokyo) 54: 7–10.
Jenkins AH, Schyns G, Potot S, Sun G, Begley TP . (2007). A new thiamin salvage pathway. Nat Chem Biol 3: 492–497.
Jurgenson CT, Begley TP, Ealick SE . (2009). The structural and biochemical foundations of thiamin biosynthesis. Annu. Rev. Biochem. 78: 569–603.
Karunakaran R, Ebert K, Harvey S, Leonard ME, Ramachandran V, Poole PS . (2006). Thiamine is synthesized by a salvage pathway in Rhizobium leguminosarum bv. viciae strain 3841. J Bacteriol 188: 6661–6668.
Koch F, Hattenrath-Lehmann TK, Goleski JA, Sanudo-Wilhelmy S, Fisher NS, Gobler CJ . (2012). Vitamin B1 and B12 uptake and cycling by plankton communities in coastal ecosystems. Front Microbiol 3: 363.
Lengeler JW, Drews G, Schlegel HG . (1999) Biology of the Prokaryotes. Wiley-Blackwell: Malden, MA, USA.
Lewin RA (ed.). (1962). Organic micronutrients. In: Physiology and Biochemistry of Algae. Academic Press: New York, NY, USA, pp 141–156.
Machlin LJ (ed.). (1984) Handbook of Vitamins: Nutritional, Biochemical, and Clinical Aspects. Marcel Dekker, Inc.: New York, NY, USA.
Martinez-Gomez N, Downs D . (2008). ThiC is an [Fe-S] cluster protein that requires AdoMet to generate the 4-amino-5-hydroxymethyl-2-methylpyrimidine moiety in thiamin synthesis. Biochemistry 47: 9054–9056.
Meyer M, Ames T, DP S, Weinberg Z, Schwalbach M, Giovannoni S et al. (2009). Identification of candidate structured RNAs in the marine organism ‘Candidatus Pelagibacter ubique’. BMC Genom 10: 268.
Morel FMM, Price NM . (2003). The biogeochemical cycles of trace metals in the oceans. Science (New York, NY) 300: 944–947.
Morris JJ, Lenski RE, Zinser ER . (2012). The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. mBio 3: e00036–12.
Morris R, Rappé MS, Connon SA, Vergin KL, Siebold WA, Carlson CA et al. (2002). SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420: 806–810.
Natarajan K . (1970). Distribution and significance of vitamin B12 and thiamine in the subarctic Pacific Ocean. Limnol Oceanogr 15: 655–659.
Natarajan K, Dugdale R . (1966). Bioassay and distribution of thiamine in the sea. Limnol Oceanogr 11: 621–629.
Natarajan KV . (1968). Distribution of thiamine, biotin, and niacin in the sea. Appl Microbiol 16: 366–369.
Norman SM, Maier VP, Echols LC . (1981). Development of a defined medium for growth of Cercospora-rosicola Passerini. Appl Environ Microbiol 41: 334–336.
Okumura K . (1961). Decomposition of thiamine and its derivatives by ultraviolet radiation. J Vitaminol (Kyoto) 24: 158–163.
Ottesen EA, Young CR, Eppley JM, Ryan JP, Chavez FP, Scholin CA et al. (2013). Pattern and synchrony of gene expression among sympatric marine microbial populations. Proceedings of the National Academy of Sciences 110: E488–E497.
Panzeca C, Tovar-Sanchez A, Agusti S, Reche I, Duarte C, Taylor G et al. (2006). B vitamins as regulators of phytoplankton dynamics. EOS Trans 87: 593–596.
Reddick JJ, Nicewonger R, Begley TP . (2001). Mechanistic studies on thiamin phosphate synthase: evidence for a dissociative mechanism. Biochemistry 40: 10095–10102.
Rippka R, Coursin T, Hess W, Lichtlé C, Scanlan DJ, Palinska KA et al. (2000). Prochlorococcus marinus Chisholm et al. 1992 subsp. pastoris subsp. nov. strain PCC 9511, the first axenic chlorophyll a2/b2-containing cyanobacterium (Oxyphotobacteria). Int J Syst Evol Microbiol 50 (Part 5): 1833–1847.
Robbertse B, Yoder RJ, Boyd A, Reeves J, Spatafora JW . (2011). Hal: an automated pipeline for phylogenetic analyses of genomic data. PLoS Curr 3: RRN1213.
Rodionov DA, Hebbeln P, Eudes A, Beek ter J, Rodionova IA, Erkens GB et al. (2008). A novel class of modular transporters for vitamins in prokaryotes. J Bacteriol 191: 42–51.
Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS . (2002). Comparative genomics of thiamin biosynthesis in procaryotes. J Biol Chem 277: 48949–48959.
Sañudo-Wilhelmy SA, Cutter LS, Durazo R, Smail EA, Gomez- Consarnau L, Webb EA et al. (2012). Multiple B-vitamin depletion in large areas of the coastal ocean. Proc Natl Acad Sci USA 109: 14041–14045.
Schauer K, Stolz J, Scherer S, Fuchs TM . (2009). Both thiamine uptake and biosynthesis of thiamine precursors are required for intracellular replication of Listeria monocytogenes. J Bacteriol 191: 2218–2227.
Sharpton TJ, Jospin G, Wu D, Langille MG, Pollard KS, Eisen JA . (2012). Sifting through genomes with iterative-sequence clustering produces a large, phylogenetically diverse protein-family resource. BMC bioinformatics 13: 264–264.
Sowell SM, Wilhelm LJ, Norbeck AD, Lipton MS, Nicora CD, Barofsky DF et al. (2009). Transport functions dominate the SAR11 metaproteome at low-nutrient extremes in the Sargasso Sea. ISME J 3: 93–105.
Stamatakis A . (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690.
Steele JA, Countway PD, Xia L, Vigil PD, Beman JM, Kim DY et al. (2011). Marine bacterial, archaeal and protistan association networks reveal ecological linkages. ISME J 5: 1414–1425.
Thrash JC, Temperton B, Swan BK, Landry ZC, Woyke T, Delong EF et al. (2014). Single-cell enabled comparative genomics of a deep ocean SAR11 bathytype. ISME J e-pub ahead of print 23 January 2014; doi:10.1038/ismej.2013.243.
Tripp HJ . (2013). The unique metabolism of SAR11 aquatic bacteria. J Microbiol 51: 147–153.
Tripp HJ, Kitner JB, Schwalbach MS, Dacey JWH, Wilhelm LJ, Giovannoni SJ . (2008). SAR11 marine bacteria require exogenous reduced sulphur for growth. Nature 452: 741–744.
Waldbauer JR, Rodrigue S, Coleman ML, Chisholm SW . (2012). Transcriptome and proteome dynamics of a light-dark synchronized bacterial cell cycle. PLoS One 7: e43432.
Webb E, Claas K, Downs D . (1998). thiBPQ encodes an ABC transporter required for transport of thiamine and thiamine pyrophosphate in Salmonella typhimurium. J Biol Chem 273: 8946–8950.
Wightman R, Meacock PA . (2003). The THI5 gene family of Saccharomyces cerevisiae: distribution of homologues among the hemiascomycetes and functional redundancy in the aerobic biosynthesis of thiamin from pyridoxine. Microbiology 149: 1447–1460.
Williams TJT, Long EE, Evans FF, Demaere MZM, Lauro FMF, Raftery MJM et al. (2012). A metaproteomic assessment of winter and summer bacterioplankton from Antarctic peninsula coastal surface waters. ISME J 6: 1883–1900.
Winkler W, Nahvi A, Breaker RR . (2002). Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419: 952–956.
Winkler WC, Breaker RR . (2005). Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol 59: 487–517.
Worden AZ, Lee J-H, Mock T, Rouze P, Simmons MP, Aerts AL et al. (2009). Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotes Micromonas. Science (New York, NY) 324: 268–272.
Wrenger C, Eschbach M-L, Müller IB, Laun NP, Begley TP, Walter RD . (2006). Vitamin B1 de novo synthesis in the human malaria parasite Plasmodium falciparum depends on external provision of 4-amino-5-hydroxymethyl-2-methylpyrimidine. Biol Chem 387: 41–51.
Wu H, Ito K, Shimoi H . (2005). Identification and characterization of a novel biotin biosynthesis gene in Saccharomyces cerevisiae. Appl Environ Microbiol 71: 6845–6855.
Zhao L, Ma X-D, Chen F-E . (2012). Development of two scalable syntheses of 4-amino-5-aminomethyl-2-methylpyrimidine: key intermediate for vitamin B1. Org Process Res Dev 16: 57–60.
Acknowledgements
This work was supported by the Gordon and Betty Moore Foundation’s Marine Microbiology Initiative and National Science Foundation grant OCE-0802004. We thank Diana Downs, Woongye Chung, Alyson Santoro, William Orsi and Jeff H Chang for useful dialogue pertinent to experimental design and manuscript preparation, Kimberly Halsey for phytoplankton cultures and manuscript revisions, Brateen Shome for synthesizing and characterizing HMP and AmMP and the crew of the R/V Atlantic Explorer for assistance during seawater collection. Mass spectrometry was performed at the Oregon State University Mass Spectrometry Facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Carini, P., Campbell, E., Morré, J. et al. Discovery of a SAR11 growth requirement for thiamin’s pyrimidine precursor and its distribution in the Sargasso Sea. ISME J 8, 1727–1738 (2014). https://doi.org/10.1038/ismej.2014.61
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2014.61
Keywords
This article is cited by
-
Ligand cross-feeding resolves bacterial vitamin B12 auxotrophies
Nature (2024)
-
Myxobacteria restrain Phytophthora invasion by scavenging thiamine in soybean rhizosphere via outer membrane vesicle-secreted thiaminase I
Nature Communications (2023)
-
Ecophysiology and genomics of the brackish water adapted SAR11 subclade IIIa
The ISME Journal (2023)
-
Vitamin B12 is not shared by all marine prototrophic bacteria with their environment
The ISME Journal (2023)
-
Evolutionary and ecological correlates of thiaminase in fishes
Scientific Reports (2023)