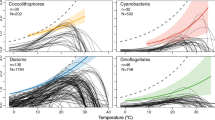
Abstract
Marine phytoplankton can evolve rapidly when confronted with aspects of climate change because of their large population sizes and fast generation times. Despite this, the importance of environment fluctuations, a key feature of climate change, has received little attention—selection experiments with marine phytoplankton are usually carried out in stable environments and use single or few representatives of a species, genus or functional group. Here we investigate whether and by how much environmental fluctuations contribute to changes in ecologically important phytoplankton traits such as C:N ratios and cell size, and test the variability of changes in these traits within the globally distributed species Ostreococcus. We have evolved 16 physiologically distinct lineages of Ostreococcus at stable high CO2 (1031±87 μatm CO2, SH) and fluctuating high CO2 (1012±244 μatm CO2, FH) for 400 generations. We find that although both fluctuation and high CO2 drive evolution, FH-evolved lineages are smaller, have reduced C:N ratios and respond more strongly to further increases in CO2 than do SH-evolved lineages. This indicates that environmental fluctuations are an important factor to consider when predicting how the characteristics of future phytoplankton populations will have an impact on biogeochemical cycles and higher trophic levels in marine food webs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Beaumont HJE, Gallie J, Kost C, Ferguson G, Rainey PB . (2009). Experimental evolution of bet hedging. Nature 462: 90–93.
Beardall J, Raven JA . (2004). The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 43: 26–40.
Chevin LM, Gallet R, Gomulkiewicz R, Holt RD, Fellous S . (2013). Phenotypic plasticity in evolutionary rescue experiments. Proc R Soc B 368: 20120089.
Christaki U, Jacquet S, Dolan JR, Vaulot D, Rassoulzadegan F . (1999). Growth and grazing on Prochlorococcus and Synechococcus by two marine ciliates. Limnol Oceangr 44: 52–61.
Collins S . (2011). Many possible worlds: expanding the ecological scenarios in experimental evolution. Evol Biol 38: 3–14.
Collins S, Rost B, Rynearson TA . (2013). Evolutionary potential of marine phytoplankton under ocean acidification. Evol Appl 7: 140–155.
Courties C, Vaquer A, Troussellier M, Lautier J, Chrétiennot-Dinet MJ, Neveux J et al. (1994). Smallest eukaryotic organism. Nature 370: 255–255.
Crawfurd KJ, Raven JA, Wheeler GL, Baxter EJ, Joint I . (2011). The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH. PLoS One 6: e26695.
Cuevas LA . (2006). Nanoheterotroph grazing on bacteria and cyanobacteria in oxic and suboxic waters in coastal upwelling areas off northern Chile. J Plank Res 28: 385–397.
Dowling DK, Simmons LW . (2009). Reactive oxygen species as universal constraints in life- history evolution. Proc R Soc B 276: 1737–1745.
Draghi JA, Whitlock MC . (2012). Phenotypic plasticity facilitates mutational variance, genetic variance, and evolvability along the major axis of environmental variation. Evolution 66: 2891–2902.
Egleston ES, Sabine CL, Morel FMM . (2010). Revelle revisited: buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity. Global Biogeochem Cycles 24: GB1002.
Falkowski PG, Barber R, Smetacek V . (1998). Biogeochemical controls and feedbacks on ocean primary production. Science 281: 200–206.
Flynn KJ, Blackford JC, Baird ME, Raven JA, Clark DR, Beardall J et al. (2012). Changes in pH at the exterior surface of plankton with ocean acidification. Nat Clim Change 2: 510–513.
Ghalambor CK, Mckay JK, Carroll SP, Reznick DN . (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21: 394–407.
Gilbert JA, Field D, Huang Y, Edwards R, Li W, Gilna P et al. (2008). Detection of large numbers of novel sequences in the metatranscriptomes of complex marine microbial communities. PLoS One 3: e3042.
Guzmán HM, la Jara Valido de A, Duarte LC, Presmanes KF . (2009). Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquacult Int 18: 189–199.
Holm-Hansen O, Riemann B . (1978). Chlorophyll a determination: improvements in methodology. Oikos 30: 438.
IPCC 2007 Intergovernmental Panel on Climate Change. (2007) Working Group I report ‘The physical science basis’. Available at http://www.ipcc.ch/report/ar5/wg1/.
Joint I, Doney SC, Karl DM . (2011). Will ocean acidification affect marine microbes? ISME J 5: 1–7.
la Jara de A, Mendoza H, Martel A, Molina C, Diaz R . (2003). Flow cytometric determination of lipid content in a marine dinoflagellate, Crypthecodinium cohnii. J Appl Physiol 15: 433–438.
Karve S, Daniel S, Chavhan Y, Anand A, Kharola SS, Dey S . (2014) E. coli populations in unpredictably fluctuating environments evolve to face novel stresses through enhanced efflux activity, bioRxiv, doi. http://dx.doi.org/10.1101/011007.
Kassen R . (2002). The experimental evolution of specialists, generalists, and the maintenance of diversity. J Evol Biol 15: 173–190.
Ketola T, Mikonranta L, Zhang J, Saarinen K, Örmälä A-M, Friman V-P et al. (2013). Fluctuating temperature leads to evolution of thermal generalism and preadaptation to novel environments. Evolution 67: 2936–2944.
Li G, Wu Y, Gao KS . (2009). Effects of Typhoon Kaemi on coastal phytoplankton assemblages in the South China Sea, with special reference to the effects of solar UV radiation. J Geophys Res 114: G04029.
Lohbeck KT, Riebesell U, Reusch TBH . (2012). Adaptive evolution of a key phytoplankton species to ocean acidification. Nat Geosci 5: 346–351.
Mariani P, Andersen KH, Visser AW, Barton AD, Kiørboe T . (2013). Control of plankton seasonal succession by adaptive grazing. Limnol Oceanogr 58: 173–184.
Marie D, Simon N, Vaulot D . (2005) Phytoplankton cell counting by flow cytometry. In Algal Culturing Techniques. Vol 1. Elsevier: Oxford, UK. pp 253–267.
Petersen TW, Brent Harrison C, Horner DN, van den Engh G . (2012). Flow cytometric characterization of marine microbes. Methods 57: 350–358.
Pinheiro JC, Bates DM . (2000) Mixed-effects models in S and S-PLUS. Statistics and Computing. Springer: New York. pp 146–174 283–479.
Riebesell U, Wolf-Gladrow DA . (1992). The relationship between physical aggregation of phytoplankton and particle flux: a numerical model. Deep Sea Res A Oceanogr Res Papers 39: 1085–1102.
Riebesell U, Bellerby RGJ, Grossart HP, Thingstad F . (2008). Mesocosm CO2 perturbation studies: from organism to community level. Biogeosciences 5: 1157–1164.
Ripa J, Olofsson H, Jonzen N . (2010). What is bet-hedging, really? Proc R Soc B. (2010) 277: 1153–1154.
Rokitta SD, John U, Rost B . (2012). Ocean acidification affects redox-balance and ion-homeostasis in the life-cycle stages of Emiliania huxleyi. PLoS One 7: e52212.
Rossoll D, Bermúdez R, Hauss H, Schulz KG, Riebesell U, Sommer U et al. (2012) Ocean acidification-induced food quality deterioration constrains trophic transfer PLoS One 7: e34737.
Schaum CE, Collins S . (2014). Plasticity predicts evolution in a marine alga. Proc Biol Sci 281: 20141486–20141486.
Schaum E, Rost B, Millar AJ, Collins S . (2013). Variation in plastic responses of a globally distributed picoplankton species to ocean acidification. Nat Clim Change 3: 298–302.
Scheinin M, Riebesell U, Rynearson T, Lohbeck K, Collins S . (2015). Experimental evolution gone wild. J R Soc Interface 12: 20150056. http://dx.doi.org/10.1098/rsif.2015.0056.
Schlüter L, Lohbeck KT, Gutowska MA, Gröger JP, Riebesell U, Reusch TBH . (2014). Adaptation of a globally important coccolithophore to ocean warming and acidification. Nat Clim Change 4: 1024–1030.
Subirana L, Pequin B, Michely S, Escande M-L, Meilland J, Derelle E et al. (2013). Morphology, genome plasticity, and phylogeny in the genus Ostreococcus. Ann Anat 164: 643–659.
Tatters AO, Schnetzer A, Fu F, Lie AYA, Caron DA, Hutchins DA . (2013). Short- versus long-term responses to changing CO2 in a coastal dinoflagellate bloom: implications for interspecific competitive interactions and community structure. Evolution 67: 1879–1891.
Thomsen J, Casties I, Pansch C, Körtzinger A, Melzner F . (2013). Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob Change Biol 19: 1017–1027.
West-Eberhard M. J . (2003) Developmental Plasticity and Evolution. Oxford University Press: New York.
Zhang Y, Klapper R, Lohbeck KT, Bach LT, Schulz KG, Reusch TBH et al. (2014). Between- and within-population variations in thermal reaction norms of the coccolithophore Emiliania huxleyi. Limnol Oceangr 59: 1570–1580.
Acknowledgements
The selection experiment and subsequent assays were conducted at the University of Edinburgh (UK). Intracellular stoichiometry was measured at the Alfred Wegener Institute Bremerhaven (Germany). The research was supported by a Royal Society (UK) University Research Fellowship (SC), by the European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007-2013), ERC grant agreement 205150 (BR) and a Scottish Universities Life Science Alliance scholarship (C-ES). We thank M Allen and ASSEMBLE (Association of European Marine Biology Laboratories), Roscoff, for providing the Ostreococcus lineages; S Rokitta, K-U Richter and U Richter for assistance in the laboratory at the AWI; M Waterfall (UoE) for assistance with flow cytometry; and JA Raven, A Millar, G Yvon-Durocher and A Buckling for providing feedback on the manuscript.
Author Contributions
ES designed and performed the experiments, analysed data and wrote the manuscript. SC designed the experiments, analysed data, wrote the manuscript and supervised the laboratory work. BR supervised the laboratory work at the Alfred Wegener institute and contributed to the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Schaum, CE., Rost, B. & Collins, S. Environmental stability affects phenotypic evolution in a globally distributed marine picoplankton. ISME J 10, 75–84 (2016). https://doi.org/10.1038/ismej.2015.102
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2015.102
This article is cited by
-
Multivariate trait analysis reveals diatom plasticity constrained to a reduced set of biological axes
ISME Communications (2021)
-
Transient exposure to novel high temperatures reshapes coastal phytoplankton communities
The ISME Journal (2020)
-
Compensation of ocean acidification effects in Arctic phytoplankton assemblages
Nature Climate Change (2018)
-
Microorganisms and ocean global change
Nature Microbiology (2017)