Abstract
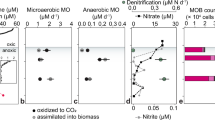
Freshwater lakes represent large methane sources that, in contrast to the Ocean, significantly contribute to non-anthropogenic methane emissions to the atmosphere. Particularly mixed lakes are major methane emitters, while permanently and seasonally stratified lakes with anoxic bottom waters are often characterized by strongly reduced methane emissions. The causes for this reduced methane flux from anoxic lake waters are not fully understood. Here we identified the microorganisms and processes responsible for the near complete consumption of methane in the anoxic waters of a permanently stratified lake, Lago di Cadagno. Interestingly, known anaerobic methanotrophs could not be detected in these waters. Instead, we found abundant gamma-proteobacterial aerobic methane-oxidizing bacteria active in the anoxic waters. In vitro incubations revealed that, among all the tested potential electron acceptors, only the addition of oxygen enhanced the rates of methane oxidation. An equally pronounced stimulation was also observed when the anoxic water samples were incubated in the light. Our combined results from molecular, biogeochemical and single-cell analyses indicate that methane removal at the anoxic chemocline of Lago di Cadagno is due to true aerobic oxidation of methane fuelled by in situ oxygen production by photosynthetic algae. A similar mechanism could be active in seasonally stratified lakes and marine basins such as the Black Sea, where light penetrates to the anoxic chemocline. Given the widespread occurrence of seasonally stratified anoxic lakes, aerobic methane oxidation coupled to oxygenic photosynthesis might have an important but so far neglected role in methane emissions from lakes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Auman AJ, Lidstrom ME . (2002). Analysis of sMMO-containing Type I methanotrophs in Lake Washington sediment. Environ Microbiol 4: 517–524.
Bahr M, Stams AJM, De la Rosa F, García-Encina PA, Muñoz R . (2011). Assessing the influence of carbón oxidation-reduction in algal-bacterial photobioreactos. Appl Microbiol Biotechnol 90: 1527–1536.
Bastviken D, Cole J, Pace M, Tranvik L . (2004). Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochem Cyc 18: 1–12.
Beal EJ, House CH, Orphan VJ . (2009). Manganese- and iron-dependent marine methane oxidation. Science 325: 184–187.
Biderre-Petit C, Jezequel D, Dugat-Bony E, Lopes F, Kuever J, Borrel G et al. (2011). Identification of microbial communities involved in the methane cycle of a freshwater meromictic lake. FEMS Microbiol Ecol 77: 533–545.
Blees J, Niemann H, Wenk CB, Zopfi J, Schubert CJ, Kirf MK et al. (2014). Micro-aerobic bacterial methane oxidation in the chemocline and anoxic water column of deep south-Alpine Lake Lugano (Switzerland). Limnol Oceanogr 59: 311–324.
Bodelier PLE, Meima-Franke M, Hordijk CA, Steenbergh AK, Hefting MM, Bodrossy L et al. (2013). Microbial minorities modulate methane consumption through niche partitioning. ISME J 7: 2214–2228.
Bossard P, Gammeter S, Lehmann C, Schanz F, Bachofen R, Bürgi HR et al. (2001). Limnological description of the Lakes Zürich, Lucerne, and Cadagno. Aquat Sci 63: 225–249.
Bowman JP . (2006). The methanotrophs - the families Methylococcacae and Methylocystaceae. In: Balows AT, Trüper HG, Dworkin M, Harder W, Schleifer KH, (eds) The Prokaryotes. Springer: Berlin, Heidelberg, Germany; New York, NY, USA, pp 266–289.
Bussmann I, Pester M, Brune A, Schink B . (2004). Preferential cultivation of type II methanotrophic bacteria from littoral sediments (Lake Constance). FEMS Microbiol Ecol 47: 179–189.
Camacho A, Erez J, Chicote A, Florin M, Squires MM, Lehmann C et al. (2001). Microbial microstratification, inorganic carbon photoassimilation and dark carbon fixation at the chemocline of the meromictic Lake Cadagno (Switzerland) and its relevance to the food web. Aquat Sci 63: 91–106.
Chen Y, Dumont MG, Cebron A, Murrell JC . (2007). Identification of active methanotrophs in a landfill cover soil through detection of expression of 16S rRNA and functional genes. Environ Microbiol 9: 2855–2869.
Cline JD . (1969). Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14: 454–458.
Costello AM, Lidstrom ME . (1999). Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol 65: 5066–5074.
Ettwig KF, Shima S, van de Pas-Schoonen KT, Kahnt J, Medema MH, op den Camp HJM et al. (2008). Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea. Environ Microbiol 10: 3164–3173.
Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MMM et al. (2010). Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464: 543–548.
Habicht KS, Miller M, Cox RP, Frigaard NU, Tonolla M, Peduzzi S et al. (2011). Comparative proteomics and activity of a green sulfur bacterium through the water column of Lake Cadagno, Switzerland. Environ Microbiol 13: 203–215.
Hales BA, Edwards C, Ritchie DA, Hall G, Pickup RW, Saunders JR . (1996). Isolation and identification of methanogen-specific DNA from blanket bog feat by PCR amplification and sequence analysis. Appl Environ Microbiol 62: 668–675.
Halm H, Musat N, Lam P, Langlois R, Musat F, Peduzzi S et al. (2009). Co-occurrence of denitrification and nitrogen fixation in a meromictic lake, Lake Cadagno (Switzerland). Environ Microbiol 11: 1945–1958.
Hanson RS, Hanson TE . (1996). Methanotrophic bacteria. Microbiol Rev 60: 439–471.
Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P et al. (2013). Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500: 567–570.
Hinrichs KU, Boetius A . (2002). The anaerobic oxidation of methane: new insights in microbial ecology and biogeochemistry. In: Wefer G, Hebbeln DB, Jørgensen BB, Schlüter M, van Weering TCE, (eds). Ocean Margin Systems. Springer-Verlag: Berlin, Germany, pp 457–477.
Holland HD . (2006). The oxygenation of the atmosphere and oceans. Phil Trans R Soc B 361: 903–915.
Holler T, Wegener G, Knittel K, Boetius A, Brunner B, Kuypers MMM et al. (2009). Substantial 13C/12C and D/H fractionation during anaerobic oxidation of methane by marine consortia enriched in vitro. Environ Microbiol Rep 1: 370–376.
Holmes AJ, Costello A, Lidstrom ME, Murrell JC . (1995). Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132: 203–208.
Holmes RM, Aminot A, Kerouel R, Hooker BA, Peterson BJ . (1999). A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56: 1801–1808.
Holtappels M, Lavik G, Jensen MM, Kuypers MMM . (2011). 15 N-Labeling experiments to dissect the contributions of heterotrophic denitrification and anammox to nitrogen removal in the OMZ waters of the Ocean. In: Klotz MG, (ed). Methods in Enzymology (Research on Nitrification and Related Processes, Part A). Academic Press: Burlington, NJ, USA, pp 223–251.
Hutchens E, Radajewski S, Dumont MG, Mcdonald IR, Murrell JC . (2004). Analysis of methanotrophic bacteria in Movile Cave by stable isotope probing. Environ Microbiol 6: 111–120.
Knittel K, Boetius A . (2009). Anaerobic oxidation of methane: progress with an unknown process. Ann Rev Microbiol 63: 311–334.
King GM . (1990). Regulation by light of methane emissions from a wetland. Nature 345: 513–515.
Kirf MK, Dinkel C, Schubert CJ, Wehrli B . (2014). Submicromolar oxygen profiles at the oxic-anoxic boundary of temperate lakes. Aquat Geochem 20: 39–57.
Lidstrom ME, Somers L . (1984). Seasonal study of methane oxidation in Lake Washington. Appl Environ Microbiol 47: 1255–1260.
Luesken FA, Zhu BL, van Alen TA, Butler MK, Diaz MR, Song B et al. (2011). pmoA primers for detection of anaerobic methanotrophs. Appl Environ Microbiol 77: 3877–3880.
McDonald IR, Kenna EM, Murrell JC . (1995). Detection of methanotrophic bacteria in environmental samples with the PCR. Appl Environl Microbiol 61: 116–121.
Milucka J, Ferdelman TG, Polerecky L, Franzke D, Wegener G, Schmid M et al. (2012). Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 491: 541–546.
Musat N, Halm H, Winterholler B, Hoppe P, Peduzzi S, Hillion F et al. (2008). A single-cell view on the ecophysiology of anaerobic phototrophic bacteria. Proc Natl Acad Sci USA 105: 17861–17866.
Nercessian O, Noyes E, Kalyuzhnaya MG, Lidstrom ME, Chistoserdova L . (2005). Bacterial populations active in metabolism of C-1 compounds in the sediment of Lake Washington, a freshwater lake. Appl Environ Microbiol 71: 6885–6899.
Noll M, Frenzel P, Conrad R . (2008). Selective stimulation of type I methanotrophs in a rice paddy soil by urea fertilization revealed by RNA-based stable isotope probing. FEMS Microbiol Ecol 65: 125–132.
Patel RN, Hoare DS . (1971). Physiological studies of methane and methanol-oxidizing bacteria - oxidation of C-1 compounds by Methylococcus capsulatus. J Bacteriol 107: 187–192.
Peduzzi S, Tonolla M, Hahn D . (2003). Vertical distribution of sulfate-reducing bacteria in the chemocline of Lake Cadagno, Switzerland, over an annual cycle. Aquat Microb Ecol 30: 295–302.
Polerecky L, Adam B, Milucka J, Musat N, Vagner T, Kuypers MMM . (2012). Look@NanoSIMS - a tool for the analysis of nanoSIMS data in environmental microbiology. Environ Microbiol 14: 1009–1023.
Powell T, Jassby A . (1974). The estimation of vertical eddy diffusivities below the thermocline in lakes. Water Resour Res 10: 191–198.
Qiu QF, Noll M, Abraham WR, Lu YH, Conrad R . (2008). Applying stable isotope probing of phospholipid fatty acids and rRNA in a Chinese rice field to study activity and composition of the methanotrophic bacterial communities in situ. ISME J 2: 602–614.
Raghoebarsing AA, Pol A, van de Pas-Schoonen KT, Smolders AJP, Ettwig KF, Rijpstra WIC et al. (2006). A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440: 918–921.
Rasigraf O, Vogt C, Richnow HH, Jetten MSM, Ettwig KF . (2012). Carbon and hydrogen isotope fractionation during nitrite-dependent anaerobic methane oxidation by Methylomirabilis oxyfera. Geochim Cosmochim Acta 89: 256–264.
Reeburgh WS, Tyler SC, Carroll J . (2006). Stable carbon and hydrogen isotope measurements on Black Sea water-column methane. Deep-Sea Res Pt II 53: 1893–1900.
Reeburgh WS . (2007). Oceanic methane biogeochemistry. Chem Rev 107: 486–513.
Rhee TS, Kettle AJ, Andreae MO . (2009). Methane and nitrous oxide emissions from the ocean: a reassessment using basin-wide observations in the Atlantic. J Geophys Res 114: 1–20.
Roslev P, King GM . (1995). Aerobic and anaerobic starvation metabolism in methanotrophic bacteria. Appl Environ Microbiol 61: 1563–1570.
Scheller S, Goenrich M, Boecher R, Thauer RK, Jaun B . (2010). The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature 465: 606–608.
Schubert CJ, Diem T, Eugster W . (2012). Methane emissions from a small wind shielded lake determined by Eddy covariance, flux chambers, anchored funnels, and boundary model calculations: a comparison. Environ Sci Technol 46: 4515–4522.
Schubert CJ, Lucas FS, Durisch-Kaiser E, Stierli R, Diem T, Scheidegger O et al. (2010). Oxidation and emission of methane in a monomictic lake (Rotsee, Switzerland). Aquat Sci 72: 455–466.
Sivan O, Adler M, Pearson A, Gelman F, Bar-Or I, John SG et al. (2011). Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol Oceanogr 56: 1536–1544.
Springer E, Sachs MS, Woese CR, Boone DR . (1995). Partial gene sequences for the a subunit of methyl-coenzyme M reductase (McrI) as a phylogenetic tool for the family Methanosarcinaceae. Int J Syst Bacteriol 45: 554–559.
Stookey LL . (1970). Ferrozine-a new spectrophotometric reagent for iron. Anal Chem 42: 779–781.
Storelli N, Peduzzi S, Saad MM, Frigaard NU, Perret X, Tonolla M . (2013). CO2 assimilation in the chemocline of Lake Cadagno is dominated by a few types of phototrophic purple sulfur bacteria. FEMS Microbiol Ecol 84: 421–432.
Tamas I, Smirnova AV, He Z, Dunfield PF . (2014). The (d)evolution of methanotrophy in the Beijerinckiaceae—a comparative genomics analysis. ISME J 8: 369–382.
Templeton AS, Chu KH, Alvarez-Cohen L, Conrad ME . (2006). Variable carbon isotope fractionation expressed by aerobic CH4-oxidizing bacteria. Geochim Cosmochim Acta 70: 1739–1752.
Tonolla M, Peduzzi R, Hahn D . (2005). Long-term population dynamics of phototrophic sulfur bacteria in the chemocline of Lake Cadagno, Switzerland. Appl Environ Microbiol 71: 3544–3550.
Trotsenko Y, Murrell JC . (2008). Metabolic aspects of aerobic obligate methanotrophy. In: Laskin AI, Sariaslani S, Gadd G, (eds). Advances in Applied Microbiology. Elsevier: Amsterdam, the Netherlands, pp 183–229.
Uhde MAC . (1992). Mischungsprozesse im hypolimnion des meromiktischen Lago di Cadagno: eine untersuchung mit hilfe natürlicher und künstlicher tracer. Unpublished MSc Thesis, Albert Ludwigs Universität Freiburg. Library Eawag: Duebendorf, Switzerland, p 90.
van der Ha D, Bundervoet B, Verstraete W, Boon N . (2011). A sustainable, carbon neutral methane oxidation by a partnership of methane oxidizing communities and microalgae. Water Res 45: 2845–2854.
van der Ha D, Nachtergaele L, Kerckhof M, Rameiyant D, Bossier P, Verstraete W et al. (2012). Conversion of biogas to bioproducts by algae and methane oxidizing bacteria. Environ Sci Technol 46: 13425–13431.
Wagener S, Schulz S, Hanselmann K . (1990). Abundance and distribution of anaerobic protozoa and their contribution to methane production in Lake Cadagno (Switzerland). FEMS Microbiol Ecol 74: 39–48.
Wang P, Wang F, Xu M, Xiao X . (2004). Molecular phylogeny of methylotrophs in a deep-sea sediment from a tropical west Pacific warm pool. FEMS Microbiol Ecol 47: 77–84.
Wankel SD, Adams MM, Johnston DT, Hansel CM, Joye SB, Girguis PR . (2012). Anaerobic methane oxidation in metalliferous hydrothermal sediments: influence on carbon flux and decoupling from sulfate reduction. Environ Microbiol 14: 2726–2740.
Wiesenburg DA, Guinasso NL . (1979). Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea-water. J Chem Eng Data 24: 356–360.
Wüest A . (1994). Interactions in lakes: biology as source of dominant physical forces. Limnologica 24: 93–104.
Acknowledgements
We thank the personnel of Piora Centro Biologia Alpina, especially Mauro Tonolla, for making it possible to use the laboratory facilities and housing. We also thank Daniela Franzke, Gabi Klockgether, Gaute Lavik, Niculina Musat, Meri Eichner, Christiana Lungu, Frank Schreiber, Marc Strous and Martin Koenneke for their assistance in the field and in the laboratory. Tim Ferdelman and David Bastviken are thanked for discussions. Martin Schmid is thanked for calculating the diffusion coefficient and Beat Müller for flux calculations. Funding has been provided by the Swiss Federal Institute of Aquatic Science (Eawag), the Max Planck Society and Swiss National Science Foundation grants (20021_135299, 20020_153091, 128707).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Milucka, J., Kirf, M., Lu, L. et al. Methane oxidation coupled to oxygenic photosynthesis in anoxic waters. ISME J 9, 1991–2002 (2015). https://doi.org/10.1038/ismej.2015.12
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2015.12
This article is cited by
-
Water column dynamics control nitrite-dependent anaerobic methane oxidation by Candidatus “Methylomirabilis” in stratified lake basins
The ISME Journal (2023)
-
The role of methanotrophy in the microbial carbon metabolism of temperate lakes
Nature Communications (2022)
-
Metabolic flexibility of aerobic methanotrophs under anoxic conditions in Arctic lake sediments
The ISME Journal (2022)
-
The possible occurrence of iron-dependent anaerobic methane oxidation in an Archean Ocean analogue
Scientific Reports (2021)
-
Niche partitioning of methane-oxidizing bacteria along the oxygen–methane counter gradient of stratified lakes
The ISME Journal (2020)