Abstract
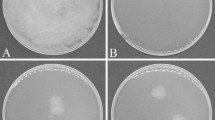
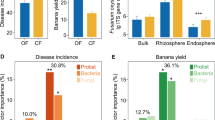
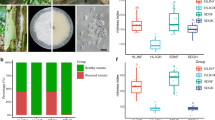
Crops lack genetic resistance to most necrotrophic pathogens. To compensate for this disadvantage, plants recruit antagonistic members of the soil microbiome to defend their roots against pathogens and other pests. The best examples of this microbially based defense of roots are observed in disease-suppressive soils in which suppressiveness is induced by continuously growing crops that are susceptible to a pathogen, but the molecular basis of most is poorly understood. Here we report the microbial characterization of a Korean soil with specific suppressiveness to Fusarium wilt of strawberry. In this soil, an attack on strawberry roots by Fusarium oxysporum results in a response by microbial defenders, of which members of the Actinobacteria appear to have a key role. We also identify Streptomyces genes responsible for the ribosomal synthesis of a novel heat-stable antifungal thiopeptide antibiotic inhibitory to F. oxysporum and the antibiotic’s mode of action against fungal cell wall biosynthesis. Both classical- and community-oriented approaches were required to dissect this suppressive soil from the field to the molecular level, and the results highlight the role of natural antibiotics as weapons in the microbial warfare in the rhizosphere that is integral to plant health, vigor and development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Altschul SF, Maddern TL, Schäffer AA, Zhang J, Zhang Z, Willer W et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25: 3389–3402.
Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G et al. (2013). Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep 30: 108–160.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA et al. (2008). The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75.
Bakker MG, Schlatter DS, Otto-Hanson L, Kinkel LL . (2014). Diffuse symbioses: roles of plant-plant, plant-microbe, and microbe-microbe interactions in structuring the soil microbiome. Mol Ecol 23: 1571–1583.
Blin K, Medema MH, Kazempour D, Fischbach MA, Breitling R, Takano E et al. (2013). antiSMASH 2.0-a versatile platform for genome mining of secondary metabolite producers. Nucl Acids Res 41: W204–W212.
Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W . (2011). Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27: 578–579.
Bowers J, Kinkel L, Jones R . (1996). Influence of disease-suppressive strains of Streptomyces on the native Streptomyces community in soil as determined by the analysis of cellular fatty acids. Can J Microbiol 42: 27–37.
Cook RJ, Rovira AD . (1976). The role of bacteria in the biological control of Gaeumannomyces graminis by suppressive soils. Soil Biol Biochem 8: 269–273.
Delcher AL, Bratke KA, Powers EC, Salzberg SL . (2007). Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23: 673–679.
Frank AM, Banderia N, Shen Z, Tanner S, Briggs SP, Smith RD et al. (2008). Clustering millions of tandem mass spectra. J Proteome Res 7: 113–122.
Giaever G, Shoemaker DD, Jones TW, Liang H, Winzeler EA, Astromoff A et al. (1999). Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat Genet 21: 278–283.
Gilbert GS . (2002). Evolutionary ecology of plant diseases in natural ecosystem. Ann Rev Phytopathol 40: 13–43.
Hartmann M, Frey B, Mayer M, Mäder P, Widmer F . (2015). Distinct soil microbial diversity under long-term organic and conventional farming. ISME J 9: 1177–1194.
Hasimoto M, Murakami T, Funahashi K, Tokunaga T, Nihei K, Okuno T et al. (2006). An RNA polymerase inhibitor, cyclothiazomycin B1, and its isomer. Bioorg Med Chem 14: 8259–8270.
Hillenmeyer ME, Fung E, Wildenhain J, Pierce SE, Hoon S, Lee W et al. (2008). The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science 320: 362–365.
Kersten RD, Yang YL, Xu Y, Cimermancic P, Nam SJ, Fenical W et al. (2011). A mass spectrometry-guided genome mining approach for natural product peptidogenomics. Nat Chem Biol 7: 794–802.
Koski LB, Gray MW, Lang BF, Burger G . (2005). AutoFACT: an automatic functional annotation and classification tool. BMC Bioinformatics 6: 151.
Kwak YS, Weller DM . (2012). Take-all of wheat and natural disease suppression: a review. Plant Pathol J 29: 125–135.
Lahdenperä M-L, Simon E, Uoti J . (1991). Mycostop® - a novel biofungicide based on Streptomyces bacteria. In: Beemster ABR, Bollen GJ, Gerlagh M, Ruissen MA, Schippers B, Tempel A (eds). Biotic Interactions and Soil-Borne Diseases. Elsevier: : Amsterdam, The Netherlands, pp 258–263.
Lee M, Han S, Chang H, Kwak YS, Weller DM, Kim D . (2013). FitSearch: a robust way to interpret a yeast fitness profile in terms of drug's mode-of-action. BMC Genomics 14: S6.
Lopez A, Parsons AB, Nislow C, Giaever G, Boone C . (2008). Chemical-genetic approaches for exploring the mode of action of natural products. Prog Drug Res 66: 239–271.
Lorang J, Anderson N, Lauer F, Wildung D . (1989). Disease decline in a Minnesota potato scab plot. Am Potato J 66: 531.
Lowe TM, Eddy SR . (1997). tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucl Acids Res 25: 955–964.
Mendes R, Kruijt M, de Brujin I, Dekkers E, vand der Voort M, Schneider JH et al. (2011). Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332: 1097–1100.
Mizuhara N, Kuroda M, Ogita A, Tanaka T, Usuki Y, Fujuta K . (2011). Antifungal thiopeptide cyclothiazomycin B1 exhibits growth inhibition accompanying morphological changes via binding to fungal cell wall chitin. Bioorg Med Chem 19: 5300–5310.
Pastor JM, Salvador M, Argandoña M, Bernal V, Reina-Bueno M, Csonka LN et al. (2010). Ectoines in cell stress protection: Uses and biotechnology production. Biotechnol Adv 28: 782–801.
Raaijmakers JM, Weller DM . (1988). Natural plant protection by 2,4-diacetylphloroglucinol-producing Pseudomonas spp. in take-all decline soils. Mol Plant Microbe Interact 11: 144–152.
Richter M, Rosselló-Móra R . (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 106: 19126–19131.
Rosenzweig N, Tiedje JM, Quensen JE III, Meng Q, Hao JJ . (2012). Microbial communities associated with scab-suppressive soils determined by pyrosequencing analyses. Plant Dis 96: 718–725.
Sanguin H, Sarniguet A, Gazengel K, Moënne-Loccoz Y, Grundmann GL . (2009). Rhizosphere bacterial communities associated with disease suppressive stages of take-all decline in wheat monoculture. New Phytol 184: 694–707.
Smith AM, Durbic T, Oh J, Urbanus M, Proctor M, Heisler LE et al. (2011). Competitive genomic screens of barcoded yeast libraries. J Vis Exp 54: e2864.
Tsai IJ, Otto TD, Berriman M . (2010). Improving draft assemblies by iterative mapping and assembly of short reads to eliminate gaps. Genome Biol 11: R41.
Wang S, Zhou S, Liu W . (2013). Opportunities and challenges from current investigations into the biosynthetic logic of nosiheptide-represented thiopeptide antibiotics. Curr Opin Chem Biol 17: 626–634.
Watrous J, Roach P, Alexandrov T, Heath BS, Yang JY, Kersten RD et al. (2012). Mass spectral molecular networking of living microbial colonies. Proc Natl Acad Sci USA 109: E1743–E1752.
Weller DM . (1988). Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu Rev Phytopathol 26: 37–407.
Weller DM . (2007). Pseudomonas biocontrol agents of soilborne pathogen: looking back over 30 years. Phytopathology 97: 250–256.
Weller DM, Raaijmakers JM, McSpadden Gardener BB, Thomashow LS . (2002). Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40: 309–348.
Yang JY, Sanchez LM, Rath CM, Liu X, Boudreau PD, Bruns N et al. (2013). Molecular networking as a dereplication strategy. J Nat Prod 76: 1686–1699.
Yu Y, Duan L, Zhang Q, Liao R, Ding Y, Pan H et al. (2009). Nosiheptide biosynthesis featuring a unique indole side ring formation on the characteristic thiopeptide framework. ACS Chem Biol 4: 855–864.
Acknowledgements
Part of this work was supported by RDA of Korea (PJ010827). Other funding included Royal Society, UK (516002.K5677/ROG) to HH, an NRF grant (NRF-2011-0017670) to JFK, NIH grant (GM97509) to BSM, BK21 PLUS to HC and S-KK and a DFG postdoctoral fellowship to MC. We thank Tim Paulitz for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Cha, JY., Han, S., Hong, HJ. et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J 10, 119–129 (2016). https://doi.org/10.1038/ismej.2015.95
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2015.95
This article is cited by
-
Characterization of rhizospheric fungi and their in vitro antagonistic potential against myco-phytopathogens invading Macrotyloma uniflorum plants
International Microbiology (2024)
-
Intercropping wheat alleviated soil acidification and suppressed Fusarium wilt of faba bean
Plant and Soil (2024)
-
Repeated exposure of wheat to the fungal root pathogen Bipolaris sorokiniana modulates rhizosphere microbiome assembly and disease suppressiveness
Environmental Microbiome (2023)
-
Diverse organic carbon activates soil microbiome functioning via niche modulation
Soil Ecology Letters (2023)
-
Harnessing Phyllosphere Microbiome for Improving Soil Fertility, Crop Production, and Environmental Sustainability
Journal of Soil Science and Plant Nutrition (2023)