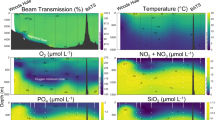
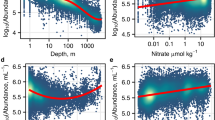
Abstract
In the bathypelagic realm of the ocean, the role of marine snow as a carbon and energy source for the deep-sea biota and as a potential hotspot of microbial diversity and activity has not received adequate attention. Here, we collected bathypelagic marine snow by gentle gravity filtration of sea water onto 30 μm filters from ~1000 to 3900 m to investigate the relative distribution of eukaryotic microbes. Compared with sediment traps that select for fast-sinking particles, this method collects particles unbiased by settling velocity. While prokaryotes numerically exceeded eukaryotes on marine snow, eukaryotic microbes belonging to two very distant branches of the eukaryote tree, the fungi and the labyrinthulomycetes, dominated overall biomass. Being tolerant to cold temperature and high hydrostatic pressure, these saprotrophic organisms have the potential to significantly contribute to the degradation of organic matter in the deep sea. Our results demonstrate that the community composition on bathypelagic marine snow differs greatly from that in the ambient water leading to wide ecological niche separation between the two environments.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Alldredge AL, Passow U, Logan BE . (1993). The abundance and significance of a class of large, transparent organic particles in the ocean. Deep-Sea Res I 40: 1131–1140.
Alldredge AL, Silver MW . (1988). Characteristics, dynamics and significance of marine snow. Prog Oceanogr 20: 41–82.
Alldredge AL, Youngbluth MJ . (1985). The significance of macroscopic aggregates (marine snow) as sites for heterotrophic bacterial production in the mesopelagic zone of the subtropical Atlantic. Deep-Sea Res 32: 1445–1456.
Anderson R, Winter C, Jürgens K . (2012). Protist grazing and viral lysis as prokaryotic mortality factors as Baltic Sea oxic-anoxic interfaces. Mar Ecol Prog Ser 467: 1–14.
Aristegui J, Gasol J, Duarte C, Herndl GJ . (2009). Microbial oceanography of the dark ocean’s pelagic realm. Limnol Oceanogr 54: 1501–1529.
Arndt H . (1993). A critical review of the importance of rhizopods (naked and testate amoebae) and actinopods (heliozoan) in lake plankton. Mar Microb Food Webs 7: 3–29.
Arndt H, Hausmann K, Wolf M . (2003). Deep-sea heterotrophic nanoflagellates of the Eastern Mediterranean Sea: qualitative and quantitative aspects of their pelagic and benthic occurrence. Mar Ecol Prog Ser 256: 45–56.
Artolozaga I, Ayo B, Latatu A, Azua I, Unanue M, Iriberri J . (2000). Spatial distribution of protists in the presence of macroaggregates in a marine system. FEMS Microbiol Ecol 33: 191–196.
Artolozaga I, Santamaria E, Lopez A, Ayo B, Iriberri J . (1997). Succession of bacterivorous protists on laboratory-made marine snow. J Plankton Res 19: 1429–1440.
Atsetsu-Scott K, Passow U . (2004). Ascending marine particles: significance of transparent exopolymer particles (TEP) in the upper ocean. Limnol Oceanogr 49: 741–748.
Bahnweg G, Sparrow FK . (1974). Four new species of thraustochytrium from Antarctic regions, with notes on the distribution of zoosporic fungi in the Antarctic marine ecosystems. Am J Bot 61: 754–766.
Baltar F, Arístegui J, Gasol JM, Sintes E, Herndl GJ . (2009). Evidence of prokaryotic metabolism on suspended particulate organic matter in the dark waters of the subtropical North Atlantic. Limnol Oceanogr 54: 182–193.
Baltar F, Arístegui J, Sintes E, Gasol JM, Reinthaler T, Herndl GJ . (2010). Significance of non-sinking particulate organic carbon and dark CO2 fixation to heterotrophic carbon demand in the mesopelagic northeast Atlantic. Geophys Res Lett 37: L09602.
Bar-Zeev E, Passow U, Castrillon SR, Elimelech M . (2015). Transparent exopolymer particles: From aquatic environments and engineered systems to membrane biofouling. Environ Sci Technol 49: 691–707.
Bochdansky AB, Bollens SM . (2004). Relevant scales in zooplankton ecology: Distribution, feeding, and reproduction of the copepod Acartia hudsonica in response to thin layers of the diatom Skeletonema costatum. Limnol Oceanogr 49: 625–636.
Bochdansky AB, Clouse MA . (2015). A new tracer to estimate community predation rates of phagotrophic protists. Mar Ecol Prog Ser 524: 55–69.
Bochdansky AB, Clouse MA, Herndl GJ . (2016). Dragon kings of the deep sea: marine particles deviate markedly from the common number-size spectrum. Sci Rep 6: 22633.
Bochdansky AB, Herndl GJ . (1992). Ecology of amorphous aggregations (marine snow) in the Northern Adriatic Sea. V. Role of fecal pellets in marine snow. Mar Ecol Prog Ser 89: 297–303.
Bochdansky AB, Huang L . (2010). Re-evaluation of the EUK516 probe for the domain Eukarya results in a suitable probe for the detection of kinetoplastids, an important group of parasitic and free-living flagellates. J Eukaryot Microbiol 57: 229–235.
Bochdansky AB, van Aken HM, Herndl GJ . (2010). Role of macroscopic particles in deep-sea oxygen consumption. Proc Natl Acad Sci 107: 8287–8291.
Boenigk J, Arndt H . (2002). Bacterivory by heterotrophic flagellates: community structure and feeding strategies. Anton Van Leeuwenhoek 81: 465–480.
Bongiorni L . (2012) Thraustochytrids, a neglected component of organic matter decomposition and food webs in marine sediments. In: Raghukumar C (ed), Biology of Marine Fungi, Progress in Molecular and Subcellular Biology. Springer-Verlag: Heidelberg, Berlin, Germany, pp 1–13.
Bratbak M . (1985). Bacterial biovolume and biomass estimations. Appl Environ Microbiol 49: 1488–1493.
Buesseler KO, Antia AN, Chen M, Fowler SW, Gardner WD, Gustafsson O et al. (2007). An assessment of the use of sediment traps for estimating upper ocean particle fluxes. J Mar Res 65: 345–416.
Buesseler KO, Boyd PW . (2009). Shedding light on processes that control particle export and flux attenuation in the twilight zone of the open ocean. Limnol Oceanogr 54: 1210–1232.
Burd AB, Hansell DA, Steinberg DK, Anderson TR, Arístegui J, Baltar F et al. (2010). Assessing the apparent imbalance between geochemical and biochemical indicators of meso- and bathypelagic biological activity: What the @$#! is wrong with present calculations of carbon budgets? Deep-Sea Res II 57: 1557–1571.
Caron DA . (1987). Grazing of attached bacteria by heterotrophic microflagellates. Microb Ecol 13: 203–218.
Caron DA, Davis PG, Madin LP, Sieburth JM . (1982). Heterotrophic bacteria and bacterivorous protozoa in oceanic macroaggregrates. Science 218: 795–797.
Cavaliere R, Ball JL, Turnbull L, Whitchurch CB . (2014). The biofilm matrix destabilizers, EDTA and DNasel, enhance the susceptibility of nontypeable Hemophilus influenza biofilms to treatment with ampicillin and ciprofloxacin. MicrobiologyOpen 3: 557–567.
Chavez-Dozal A, Gorman C, Erken M, Steinberg PD, McDougald D, Nishiguchi MK . (2013). Predation response of Vibrio fischeri biofilms to bacterivorus protists. Appl Environ Microbiol 79: 553–558.
Chenu C, Stotzky G . (2002) Interactions between microorganisms and soil particles: an overview. In: Huang PM, Bollag J-M, Senesi N (eds), Interactions between Soil Particles and Microorganisms. John Wiley and Sons: New York, pp 3–40.
Church MJ . (2008) Resource control of bacterial dynamics in the sea. In: Kirchman DL (ed), Microbial Ecology of the Oceans, 2nd edn, John Wiley & Sons, Inc.: Hoboken, NJ, USA, pp 335–382.
Ciobanu M-C, Burgaud G, Dufresne A, Breuker A, Rédou V, Maamar SB et al. (2014). Microorganisms persist at record depths in the subseafloor of the Canterbury Basin. ISME J 8: 1370–1380.
Clipson N, Otte M, Landy E . (2006) . Biogeochemical roles of fungi in marine and estuarine habitats. In: Gadd GM (ed.). Fungi in Biogeochemical Cycles. Cambridge University Press: CA, USA, pp 436–461.
Countway PD, Gast RJ, Savai P, Caron DA . (2005). Protistan diversity estimates based on 18S rDNA from seawater incubations in the western North Atlantic. J Eukaryot Microbiol 52: 95–106.
Damare V, Raghukumar S . (2010). Association of the stramenopilan protists, the aplanochytrids, with zooplankton of the equatorial Indian Ocean. Mar Ecol Prog Ser 399: 53–68.
Damare V, Raghukumar S . (2008). Abundance of thraustochytrids and bacteria in the equatorial Indian Ocean, in relation to transparent exopolymeric particles (TEP). FEMS Microbiol Ecol 65: 40–49.
de Vries FT, Hoffland E, van Eekeren N, Brussard L, Bloem J . (2006). Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol Biochem 38: 2092–2103.
Ducklow HW, Carlson CA . (1992). Oceanic bacterial production. In: Marshall KC (ed.). Advances in Microbial Ecology. Plenum Press: New York, pp 113–181.
Edgcomb VP, Beaudoin D, Gast R, Biddle JF, Teske A . (2011a). Marine subsurface eukaryotes: the fungal majority. Environ Microbiol 13: 172–183.
Edgcomb VP, Orsi W, Bunge J, Jeon S, Christen R, Leslin C et al. (2011b). Protistan microbial observatory in the Cariaco Basin, Caribbean. I. Pyrosequencing vs Sanger insights into species richness. ISME J 5: 1344–1356.
Eloe EA, Fadrosh DW, Novotny M, Zeigler Allen L, Kim M, Lombardo M-J et al. (2011). Going deeper: Metagenome of a hadopelagic microbial community. PLoS One 6: e20388.
Fontanez KM, Eppley JM, Samo TJ, Karl DM, Delong EF . (2015). Microbial community structure and function on sinking particles in the North Pacific Subtropical Gyre. Front Microbiol 6: 469.
Frey-Klett P, Burlinson P, Deveau A, Barret M, Tarkka M, Sarniguet A . (2011). Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol Mol Biol Rev 75: 583–609.
Fuhrman JA, Noble RT . (1995). Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol Oceanogr 40: 1236–1242.
Fukuda R, Ogawa H, Nagata T, Koike I . (1998). Direct determination of carbon and nitrogen contents of natural bacterial assemblages in marine environments. Appl Environ Microbiol 64: 3352–3358.
Gaertner A . (1982). Lower marine fungi from the Northwest African upwelling areas and from the Atlantic off Portugal. Meteor Forsch Ergebn D 34: 9–30.
Gasol JM, del Giorgio PA, Duarte DM . (1997). Biomass distribution in marine planktonic communities. Limnol Oceanogr 42: 1353–1363.
Gutiérrez MH, Pantoja S, Tejos E, Quiñones RA . (2011). The role of fungi in processing marine organic matter in the upwelling ecosystem off Chile. Mar Biol 158: 205–219.
Hassett BT, Gradinger R . (2016). Chytrids dominate arctic marine fungal communities. Environ Microbiol 18: 2001–2009.
Herndl GJ, Reinthaler T . (2013). Microbial control of the dark end of the biological pump. Nat Geosci 6: 718–724.
Honjo S, Manganini SJ, Krishfield RA, Francois R . (2008). Particulate organic carbon fluxes to the ocean interior and factors controlling the biological pump: a synthesis of global sediment trap programs since 1983. Prog Oceanogr 76: 217–285.
Hugenholtz P, Tyson GW, Blackall L . (2001). Design and evaluation of 16S rRNA-targeted oligonucleotide probes for fluorescence in situ hybridization Lieberman BA . (ed), Methods in Molecular Biology, vol. 176. Steroid Receptor Methods: Protocols and Assays Humana Press Inc: Totowa, NJ, pp 29–41.
Hwang J, Druffel ERM, Bauer JE . (2006). Incorporation of aged dissolved organic carbon (DOC) by oceanic particulate organic carbon (POC): an experimental approach using natural carbon isotopes. Mar Chem 98: 315–322.
Jobard M, Rasconi S, Sime-Ngando T . (2010). Diversity and functions of microscopic fungi: A missing component in pelagic food webs. Aquat Sci 72: 255–268.
Kimura H, Fukuba T, Naganuma T . (1999). Biomass of the thraustochytrid protoctists in coastal water. Mar Ecol Prog Ser 189: 27–33.
Kirchman DL . (2008) Introduction and overview. In: Kirchman DL (ed.). Microbial Ecology of the Oceans, 2nd edn.pp 1–26.
Koeve W, Ducklow HW . (2001). JGOFS research in the North Atlantic Ocean: a decade of research, synthesis and modelling. Deep-Sea Res II 48: 2141–2424.
Koid A, Nelson WC, Mraz A, Heidelberg KB . (2012). Comparative analysis of eukaryotic marine microbial assemblages from 18S rRNA gene and gene transcript clone libraries by using different methods of extraction. Appl Environ Microbiol 78: 3958–3965.
Kutty SN, Philip R . (2009). Marine yeasts - a review. Yeast 25: 465–483.
Lara E, Moreira D, Vereshchaka A, Lopez-Garcia P . (2009). Pan-oceanic distribution of new highly diverse clades of deep-sea diplonemids. Environ Microbiol 11: 47–55.
Le Calvez T, Burgaud G, Mahé S, Barbier G, Vandenkoornhuyse P . (2009). Fungal diversity in deep-sea hydrothermal ecosystems. Appl Environ Microbiol 75: 6415–6421.
Lloyd M . (1967). Mean crowding. J Anim Ecol 36: 1–30.
López-Garcí aP, Rodríguez-Valera F, Pedrós-Alió C, Moreira D . (2001). Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature 409: 603–607.
Lukeš J, Flegontova O, Horák A . (2015). Quick guide: Diplonemids. Current Biol 25: R1–R3.
Martin JH, Knauer GA, Karl DM, Broenkow WW . (1987). VERTEX: carbon cycling in the northeast Pacific. Deep-Sea Res 34: 267–285.
Menden-Deuer S, Lessard EJ . (2000). Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45: 569–579.
Morgan-Smith D, Clouse MA, Herndl GJ, Bochdansky AB . (2013). Diversity and distribution of microbial eukaryotes in the deep tropical and subtropical North Atlantic Ocean. Deep-Sea Res I 78: 58–69.
Mukherjee I, Hodoki Y, Nakano S . (2015). Kinetoplastid flagellates overlooked by universal primers dominate in the oxygenated hypolimnion of Lake Biwa, Japan. FEMS Microbiol Ecol 91: 1–11.
Newell SY . (1994). Ecomethodology for organoosmotrophs: prokaryotic unicellular versus eukaryotic mycelial. Microb Ecol 28: 151–157.
Newell SY, Statzell-Tallman A . (1982). Factors for conversion of fungal biovolume values to biomass, carbon and nitrogen: variation with mycelial ages, growth conditions, and strains of fungi from a salt marsh. OIKOS 39: 261–268.
Orsi WD, Edgcomb VP, Christman GD, Biddle JF . (2013). Gene expression in the deep biosphere. Nature 499: 205–209.
Orsi WD, Smith JM, Liu S, Liu Z, Sakamato CM, Wilken S et al. (2016). Diverse, uncultivated bacteria and archaea underlying the cycling of dissolved protein in the ocean. ISME J e-pub ahead of print 8 March 2016; doi: sdoi:10.1038/ismej.2016.20.
Pernice MC, Giner CR, Logares R, Perera-Bel J, Acinas SG, Duarte CM et al. (2016). Large variability of bathypelagic microbial eukaryotic communities across the world's oceans. ISME J 10: 945–958.
Pernice MC, Irene Forn I, Gomes A, Lara E, Alonso-Sáez L, Arrieta JM et al. (2015). Global distribution of planktonic heterotrophic protists in the deep ocean. ISME J 9: 782–792.
Raghukumar S . (2002). Ecology of the marine protists, the Labyrinthulomycetes (Thraustochytrids and Labyrinthulids). Eur J Protistol 38: 127–145.
Raghukumar S, Raghukumar C . (1999). Thraustochytrid fungoid protists in faecal pellets of the tunicate Pegea confoedereta, their tolerance to deep-sea conditions and implication in degradation processes. Mar Ecol Prog Ser 190: 133–140.
Rédou V, Navarri M, Meslet-Cladière L, Barbier G, Burgaud G . (2015). Species richness and adaptation of marine fungi from deep-subseafloor sediments. Appl Environ Microbiol 81: 3571–3583.
Richards TA, Leonard G, Mahé F, del Campo J, Romac S, Jones MDM et al. (2015). Molecular diversity and distribution of marine fungi across 130 European environmental samples. Proc Biol Sci 282: pii: 20152243.
Riemann F, Schaumann K . (1993). Thraustochytrid protists in Antarctic fast ice? Antarctic Sci 5: 279–280.
Riemann F, Schrage M . (1983). On a mass occurrence of a thraustochytrid protist (fungi or rhizopodan protozoa) in an Antarctic anaerobic marine sediment. Veröff Inst Meeresforsch Bremerh 19: 191–202.
Rogerson A, Anderson OR, Vogel C . (2003). Are planktonic naked amoebae predominantly floc associated or free in the water column? J Plankton Res 25: 1359–1365.
Salazar G, Cornejo-Castillo FM, Borrull E, Díez-Vives C, Lara E, Vaqué D et al. (2015). Particle-association lifestyle is a phylogenetically conserved trait in bathypelagic prokaryotes. Mol Ecol 24: 5692–5706.
Sheridan CC, Steinberg DK, Kling GW . (2002). The microbial and metazoan community associated with colonies of Trichodesmium spp.: a quantitative survey. J Plankton Res 24: 913–922.
Silver M . (2015). Marine snow: a brief historical sketch. Limnol Oceanogr Bull 24: 5–10.
Silver MW, Alldredge AL . (1981). Bathypelagic marine snow: deep-sea algal and detrital community. J Mar Res 39: 501–530.
Silver MW, Shanks AL, Trent JD . (1978). Marine snow: microplankton habitat and source of small-scale patchiness in pelagic populations. Science 201: 371–373.
Stokes NA, Ragone Calvo LM, Reece KS, Burreson EM . (2002). Molecular diagnostics, field validation, and phylogenetic analysis of Quahog Parasite Unknown (QPX), a pathogen of the hard clam Mercenaria mercenaria. Dis Aquat Organ 52: 233–247.
Takishita K, Tsuchiya M, Reimer JD, Maruyama T . (2006). Molecular evidence demonstrating the basidiomycetous fungus Cryptococcus curvatus is the dominant microbial eukaryote in sediment at the Kuroshima Knoll methane seep. Extremophiles 10: 165–169.
Taylor JD, Cunliffe M . (2016). Multi-year assessment of coastal planktonic fungi reveals environmental drivers of diversity and abundance. ISME J e-pub ahead of print 4 March 2016; doi: doi:10.1038/ismej.2016.24.
Teira E, Reinthaler T, Pernthaler A, Pernthaler J, Herndl GJ . (2004). Combining catalyzed reporter deposition-fluorescence in situ hybridization and microautoradiography to detect substrate utilization by bacteria and archaea in the deep ocean. Appl Environ Microbiol 70: 4411–4414.
Tisthammer KH, Cobian GM, Amend AS . (2016). Global biogeography of marine fungi is shaped by the environment. Fungal Ecol 19: 39–46.
Turley CM, Lochte K, Patterson DJ . (1988). A barophilic flagellate isolated from 4500 m in the mid-North Atlantic. Deep-Sea Res 35: 1079–1092.
van Aken HM . (2000). The hydrography of the mid-latitude northeast Atlantic Ocean I: the deep-water masses. Deep-Sea Res I 47: 757–788.
van Veen JA, Paul EA . (1979). Conversion of biovolume measurements of soil organisms, grown under various moisture tensions, to biomass and their nutrient content. Appl Environ Microbiol 37: 686–692.
Wang G, Wang X, Liu X, Li Q . (2012) Diversity and biogeochemical function of planktonic fungi in the ocean. In: Raghukumar C (ed), Biology of Marine Fungi. Progress in Molecular and Subcellular Biology. Springer-Verlag: Heidelberg, Berlin, Germany, Vol. 53, pp 71–88.
Acknowledgements
We thank the crew of the RV Pelagia. We are grateful to Uta Passow (University of California, Santa Barbara) for suggesting the use of EDTA to make accumulated TEP on the 30 μm filters sufficiently porous for DAPI and CARD-FISH probes. This study was funded by the National Science Foundation project #1235169 to ABB and by the European Research Council under the European Community’s Seventh Framework Program (FP7/2007–2013)/ERC grant agreement No. 268595 (MEDEA project) to GJH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Bochdansky, A., Clouse, M. & Herndl, G. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J 11, 362–373 (2017). https://doi.org/10.1038/ismej.2016.113
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2016.113
This article is cited by
-
Organic matter degradation by oceanic fungi differs between polar and non-polar waters
Nature Communications (2025)
-
Size-fractionated fungal communities in the sunlit ocean
Communications Biology (2025)
-
Subtropical coastal microbiome variations due to massive river runoff after a cyclonic event
Environmental Microbiome (2024)
-
A fungi hotspot deep in the ocean: explaining the presence of Gjaerumia minor in equatorial Pacific bathypelagic waters
Scientific Reports (2024)
-
Disentangling microbial networks across pelagic zones in the tropical and subtropical global ocean
Nature Communications (2024)