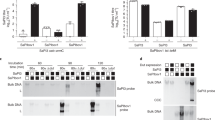
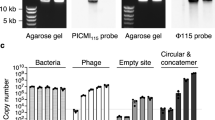
Abstract
The SaPIs are a cohesive subfamily of extremely common phage-inducible chromosomal islands (PICIs) that reside quiescently at specific att sites in the staphylococcal chromosome and are induced by helper phages to excise and replicate. They are usually packaged in small capsids composed of phage virion proteins, giving rise to very high transfer frequencies, which they enhance by interfering with helper phage reproduction. As the SaPIs represent a highly successful biological strategy, with many natural Staphylococcus aureus strains containing two or more, we assumed that similar elements would be widespread in the Gram-positive cocci. On the basis of resemblance to the paradigmatic SaPI genome, we have readily identified large cohesive families of similar elements in the lactococci and pneumococci/streptococci plus a few such elements in Enterococcus faecalis. Based on extensive ortholog analyses, we found that the PICI elements in the four different genera all represent distinct but parallel lineages, suggesting that they represent convergent evolution towards a highly successful lifestyle. We have characterized in depth the enterococcal element, EfCIV583, and have shown that it very closely resembles the SaPIs in functionality as well as in genome organization, setting the stage for expansion of the study of elements of this type. In summary, our findings greatly broaden the PICI family to include elements from at least three genera of cocci.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J et al. (2001). The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res 11: 731–753.
Carpena N, Manning KA, Dokland T, Marina A, Penadés JR . (2016). Convergent evolution of pathogenicity islands in helper cos phage interference. Philos Trans R Soc Lond B 371: 20150505.
Charpentier E, Anton AI, Barry P, Alfonso B, Fang Y, Novick RP . (2004). Novel cassette-based shuttle vector system for gram-positive bacteria. Appl Environ Microbiol 70: 6076–6085.
Chen J, Novick RP . (2009). Phage-mediated intergeneric transfer of toxin genes. Science 323: 139–141.
Chen J, Carpena N, Quiles-Puchalt N, Ram G, Novick RP, Penadés JR . (2015a). Intra- and inter-generic transfer of pathogenicity island-encoded virulence genes by cos phages. ISME J 9: 1260–1263.
Chen J, Ram G, Penadés JR, Brown S, Novick RP . (2015b). Pathogenicity island-directed transfer of unlinked chromosomal virulence genes. Mol Cell 57: 138–149.
Chopin A, Bolotin A, Sorokin A, Ehrlich SD, Chopin M . (2001). Analysis of six prophages in Lactococcus lactis IL1403: different genetic structure of temperate and virulent phage populations. Nucleic Acids Res 29: 644–651.
Claverys J-P, Martin B, Håvarstein LS . (2007). Competence-induced fratricide in streptococci. Mol Microbiol 64: 1423–1433.
Duerkop BA, Clements CV, Rollins D, Rodrigues JLM, Hooper LV . (2012). A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc Natl Acad Sci USA 109: 17621–17626.
Ferrer MD, Quiles-Puchalt N, Harwich MD, Tormo-Más MÁ, Campoy S, Barbé J et al. (2011). RinA controls phage-mediated packaging and transfer of virulence genes in Gram-positive bacteria. Nucleic Acids Res 39: 5866–5878.
Frígols B, Quiles-Puchalt N, Mir-Sanchis I, Donderis J, Elena SF, Buckling A et al. (2015). Virus satellites drive viral evolution and ecology. PLoS Genet 11: e1005609.
Hamilton CM, Aldea M, Washburn BK, Babitzke P, Kushner SR . (1989). New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol 171: 4617–4622.
Kanehisa M, Goto S . (2000). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28: 27–30.
Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP . (1998). The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol 29: 527–543.
Maiques E, Ubeda C, Tormo MA, Ferrer MD, Lasa I, Novick RP et al. (2007). Role of staphylococcal phage and SaPI integrase in intra- and interspecies SaPI transfer. J Bacteriol 189: 5608–5616.
Matos RC, Lapaque N, Rigottier-Gois L, Debarbieux L, Meylheuc T, Gonzalez-Zorn B et al. (2013). Enterococcus faecalis prophage dynamics and contributions to pathogenic traits. PLoS Genet 9: e1003539.
Nguyen SV, McShan WM . (2014). Chromosomal islands of Streptococcus pyogenes and related streptococci: molecular switches for survival and virulence. Front Cell Infect Microbiol 4: 109.
Novick RP, Ram G . (2015). The floating (pathogenicity) island: a genomic dessert. Trends Genet 32: 114–126.
Novick RP, Christie GE, Penadés JR . (2010). The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol 8: 541–551.
O'Callaghan CH, Morris A, Kirby SM, Shingler AH . (1972). Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother 1: 283–288.
Paulsen IT, Banerjei L, Myers GSA, Nelson KE, Seshadri R, Read TD et al. (2003). Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299: 2071–2074.
Penadés JR, Christie GE . (2015). The phage-inducible chromosomal islands: a family of highly evolved molecular parasites. Annu Rev Virol 2: 181–201.
Quiles-Puchalt N, Carpena N, Alonso JC, Novick RP, Marina A, Penadés JR . (2014). Staphylococcal pathogenicity island DNA packaging system involving cos-site packaging and phage-encoded HNH endonucleases. Proc Natl Acad Sci USA 111: 6016–6021.
Quiles-Puchalt N, Tormo-Más MÁ, Campoy S, Toledo-Arana A, Monedero V, Lasa I et al. (2013). A super-family of transcriptional activators regulates bacteriophage packaging and lysis in Gram-positive bacteria. Nucleic Acids Res 41: 7260–7275.
Ram G, Chen J, Kumar K, Ross HF, Ubeda C, Damle PK et al. (2012). Staphylococcal pathogenicity island interference with helper phage reproduction is a paradigm of molecular parasitism. Proc Natl Acad Sci USA 109: 16300–16305.
Ram G, Chen J, Ross HF, Novick RP . (2014). Precisely modulated pathogenicity island interference with late phage gene transcription. Proc Natl Acad Sci USA 111: 14536–14541.
Ruzin A, Lindsay J, Novick RP . (2001). Molecular genetics of SaPI1—a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol 41: 365–377.
Scott J, Nguyen SV, King CJ, Hendrickson C, McShan WM . (2012). Phage-like Streptococcus pyogenes chromosomal islands (SpyCI) and mutator phenotypes: control by growth state and rescue by a SpyCI-encoded promoter. Front Microbiol 3: 317.
Scott J, Thompson-Mayberry P, Lahmamsi S, King CJ, McShan WM . (2008). Phage-associated mutator phenotype in group A streptococcus. J Bacteriol 190: 6290–6301.
Straume D, Stamsås GA, Håvarstein LS . (2015). Natural transformation and genome evolution in Streptococcus pneumoniae. Infect Genet Evol 33: 371–380.
Tormo-Más MÁ, Donderis J, García-Caballer M, Alt A, Mir-Sanchis I, Marina A et al. (2013). Phage dUTPases control transfer of virulence genes by a proto-oncogenic G protein-like mechanism. Mol Cell 49: 947–958.
Tormo MA, Ferrer MD, Maiques E, Ubeda C, Selva L, Lasa I et al. (2008). Staphylococcus aureus pathogenicity island DNA is packaged in particles composed of phage proteins. J Bacteriol 190: 2434–2440.
Tormo-Más MÁ, Mir I, Shrestha A, Tallent SM, Campoy S, Lasa I et al. (2010). Moonlighting bacteriophage proteins derepress staphylococcal pathogenicity islands. Nature 465: 779–782.
Ubeda C, Barry P, Penadés JR, Novick RP . (2007a). A pathogenicity island replicon in Staphylococcus aureus replicates as an unstable plasmid. Proc Natl Acad Sci USA 104: 14182–14188.
Ubeda C, Maiques E, Barry P, Matthews A, Tormo MA, Lasa I et al. (2008). SaPI mutations affecting replication and transfer and enabling autonomous replication in the absence of helper phage. Mol Microbiol 67: 493–503.
Ubeda C, Maiques E, Knecht E, Lasa I, Novick RP, Penadés JR . (2005). Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol 56: 836–844.
Ubeda C, Maiques E, Tormo MA, Campoy S, Lasa I, Barbé J et al. (2007b). SaPI operon I is required for SaPI packaging and is controlled by LexA. Mol Microbiol 65: 41–50.
Ubeda C, Tormo MA, Cucarella C, Trotonda P, Foster TJ, Lasa I et al. (2003). Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol Microbiol 49: 193–210.
Viana D, Blanco J, Tormo-Más MÁ, Selva L, Guinane CM, Baselga R et al. (2010). Adaptation of Staphylococcus aureus to ruminant and equine hosts involves SaPI-carried variants of von Willebrand factor-binding protein. Mol Microbiol 77: 1583–1594.
Acknowledgements
This work was supported by Grants BIO2011-30503-C02-01, Eranet-pathogenomics PIM2010EPA-00606 and Consolider-Ingenio CSD2009-00006 from the Ministerio de Ciencia e Innovación (MICINN), and Grant MR/M003876/1 (Medical Research Council, UK) (to JRP), and by National Institutes of Health Grant R01AI022159 (to RPN and JRP).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Martínez-Rubio, R., Quiles-Puchalt, N., Martí, M. et al. Phage-inducible islands in the Gram-positive cocci. ISME J 11, 1029–1042 (2017). https://doi.org/10.1038/ismej.2016.163
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2016.163
This article is cited by
-
Genetics, ecology and evolution of phage satellites
Nature Reviews Microbiology (2025)
-
Anti-phage defence through inhibition of virion assembly
Nature Communications (2024)
-
Tail assembly interference is a common strategy in bacterial antiviral defenses
Nature Communications (2024)
-
Phage-inducible chromosomal minimalist islands (PICMIs), a novel family of small marine satellites of virulent phages
Nature Communications (2024)
-
Engineered phage enzymes against drug-resistant pathogens: a review on advances and applications
Bioprocess and Biosystems Engineering (2024)