Abstract
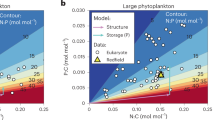
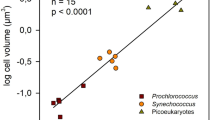
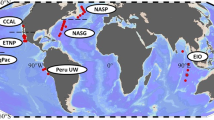
The factors that control elemental ratios within phytoplankton, like carbon:nitrogen:phosphorus (C:N:P), are key to biogeochemical cycles. Previous studies have identified relationships between nutrient-limited growth and elemental ratios in large eukaryotes, but little is known about these interactions in small marine phytoplankton like the globally important Cyanobacteria. To improve our understanding of these interactions in picophytoplankton, we asked how cellular elemental stoichiometry varies as a function of steady-state, N- and P-limited growth in laboratory chemostat cultures of Synechococcus WH8102. By combining empirical data and theoretical modeling, we identified a previously unrecognized factor (growth-dependent variability in cell size) that controls the relationship between nutrient-limited growth and cellular elemental stoichiometry. To predict the cellular elemental stoichiometry of phytoplankton, previous theoretical models rely on the traditional Droop model, which purports that the acquisition of a single limiting nutrient suffices to explain the relationship between a cellular nutrient quota and growth rate. Our study, however, indicates that growth-dependent changes in cell size have an important role in regulating cell nutrient quotas. This key ingredient, along with nutrient-uptake protein regulation, enables our model to predict the cellular elemental stoichiometry of Synechococcus across a range of nutrient-limited conditions. Our analysis also adds to the growth rate hypothesis, suggesting that P-rich biomolecules other than nucleic acids are important drivers of stoichiometric variability in Synechococcus. Lastly, by comparing our data with field observations, our study has important ecological relevance as it provides a framework for understanding and predicting elemental ratios in ocean regions where small phytoplankton like Synechococcus dominates.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bertilsson S, Berglund O, Karl DM, Chisholm SW . (2003). Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol Oceanogr 48: 1721–1731.
Bonachela JA, Allison SD, Martiny AC, Levin SA . (2013). A model for variable phytoplankton stoichiometry based on cell protein regulation. Biogeosciences 10: 4341–4356.
Claquin P, Martin-jézéquel V, Kromkamp JC, Veldhuis MJW, Kraay GW . (2002). Uncoupling of silicon compared with carbon and nitrogen metabolisms and the rol of the cell cycle in continuous cultures of Thalassiosira pseudonana (Bacillariophyceae) under light, nitrogen, and phosphorus control. J Phycol 38: 922–930.
Cook JR . (1963). Adaptations in growth and division in euglena effected by energy supply. J Protozool 10: 436–444.
Daines SJ, Clark JR, Lenton TM . (2014). Multiple environmental controls on phytoplankton growth strategies determine adaptive responses of the N: P ratio. Ecol Lett 17: 414–425.
DeVries T, Deutsch C . (2014). Large-scale variations in the stoichiometry of marine organic matter respiration. Nat Geosci 7: 890–894.
Droop MR . (1968). Vitamin B12 and marine ecology. IV. The kinetics of uptake, growth and inhibtition in Monochrysis lutheri. J Mar Biol Ass UK 48: 689–733.
Dyhrman ST, Palenik B . (2001). A single-cell immunoassay for phosphate stress in the dinoflagellate Prorocentrum minimum (Dinophyceae). J Phycol 37: 400–410.
Elser JJ, O’Brien WJ, Dobberfuhl DR, Dowling TE . (2000). The evolution of ecosystem processes: growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. J Evol Biol 13: 845–853.
Elser JJ, Sterner RW, Gorokhova E, Fagan WF, Markow TA, Cotner JB et al. (2000). Biological stoichiometry from genes to ecosystems. Ecol Lett 3: 540–550.
Finkel ZV, Beardall J, Flynn KJ, Quigg A, Rees TAV, Raven JA . (2010). Phytoplankton in a changing world: cell size and elemental stoichiometry. J Plankton Res 32: 119–137.
Flombaum P, Gallegos JL, Gordillo RA, Rincón J, Zabala LL, Jiao N et al. (2013). Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc Natl Acad Sci USA 110: 9824–9829.
Fu FX, Warner ME, Zhang Y, Feng Y, Hutchins DA . (2007). Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria). J Phycol 43: 485–496.
Galbraith ED, Martiny AC . (2015). A simple nutrient-dependence mechanism for predicting the stoichiometry of marine ecosystems. Proc Natl Acad Sci USA 112: 201423917.
Geider R, La Roche J . (2002). Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol 37: 1–17.
Geider RJ, MacIntyre HL, Kana TM . (1998). A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol Oceanogr 43: 679–694.
Goldman JC, McCarthy JJ, Peavey DG . (1979). Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 279: 210–215.
Healey F . (1985). Interacting effects of light and nutrient limitation on the growth rate of Synechococcus linearis (Cyanophyceae). J Phycol 21: 134–146.
Heldal M, Scanlan DJ, Norland S, Thingstad F, Mann NH . (2003). Elemental composition of single cells of various strains of marine Prochlorococcus and Synechococcus using X-ray microanalysis. Limnol Oceanogr 48: 1732–1743.
Ho T-Y, Quigg A, Finkel ZV, Milligan AJ, Wyman K, Falkowski PG et al. (2003). The elemental composition of some marine phytoplankton. J Phycol 39: 1145–1159.
Klausmeier CA, Litchman E, Daufresne T, Levin SA . (2004a). Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429: 171–174.
Klausmeier CA, Litchman E, Daufresne T, Levin SA . (2008). Phytoplankton stoichiometry. Ecol Res 23: 479–485.
Klausmeier CA, Litchman E, Levin SA . (2004b). Phytoplankton growth and stoichiometry under multiple nutrient limitation. Limnol Oceanogr 49: 1463–1470.
Kretz CB, Bell DW, Lomas DA, Lomas MW, Martiny AC . (2015). Influence of growth rate on the physiological response of marine Synechococcus to phosphate limitation. Front Microbiol 6: 6–11.
Laws EA, Bannister TT . (1980). Nutrient- and light-limited growth of Thalassiosira fluviatilis in continuous culture, with implications for phytoplankton growth in the ocean. Limnol Oceanogr 25: 457–473.
Legović T, Cruzado A . (1997). A model of phytoplankton growth on multiple nutrients based on the Michaelis-Menten-Monod uptake, Droop’s growth and Liebig's law. Ecol Modell 99: 19–31.
Lehman JT, Botkin DB, Likens GE . (1975). The assumptions and rationales of a computer model of phytoplankton dynamics. Limnol Oceanogr 20: 343–364.
Lomas MW, Burke AL, Lomas DA, Bell DW, Shen C, Dyhrman ST et al. (2010). Sargasso Sea phosphorus biogeochemistry: an important role for dissolved organic phosphorus (DOP). Biogeosciences 7: 695–710.
Lourenço SO, Barbarino E, Marquez UML, Aidar E . (1998). Distribution of intracellular nitrogen in marine microalgae: Basis for the calculation of specific nitrogen-to-protein conversion factors. J Phycol 34: 798–811.
Marriot ID, Edwin AD, Rowley BI . (1981). Effect of growth rate and nutrient limitation on the adenine nucleotide content, energy charge and enzymes of adenylate metabolism in Azotobacter beijerinckii. J Gen Microbiol 125: 375–382.
Martin P, Dyhrman ST, Lomas MW, Poulton NJ, Van Mooy BAS . (2014). Accumulation and enhanced cycling of polyphosphate by Sargasso Sea plankton in response to low phosphorus. Proc Natl Acad Sci USA 111: 8089–8094.
Martiny AC, Pham CTA, Primeau FW, Vrugt JA, Moore JK, Levin SA et al. (2013). Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat Geosci 6: 279–283.
Michaels A, Dow R, Elardo K . (1997a). The determination of phosphorus in seawater. In: Bermuda Atlantic Time-Series Study Methods. JGOFS Planning Office: Woods Hole, MA, USA, pp 71–74.
Michaels A, Dow R, Howse F . (1997b). The determination of nitrate in seawater. In: Bermuda Atlantic Time-series Study Methods. JGOFS Planning Office: Woods Hole, MA, USA, pp 61–66.
Moore CM, Mills MM, Achterberg EP, Geider RJ, LaRoche J, Lucas MI et al. (2009). Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat Geosci 2: 867–871.
Moore CM, Mills MM, Arrigo KR, Berman-Frank I, Bopp L, Boyd PW et al. (2013). Processes and patterns of oceanic nutrient limitation. Nat Geosci 6: 701–710.
Moore LR, Goericke R, Chisholm S . (1995). Comparative physiology of Synechococcus and Prochlorococcus: influence of light and temperature on the growth, pigments, fluorescence and absorptive properties. Mar Ecol Prog Ser 116: 259–275.
Morel FMM . (1987). Kinetics of nutrient uptake and growth in phytoplankton. J Phycol 23: 137–150.
Mouginot C, Zimmerman AE, Bonachela JA, Fredricks H, Allison SD, Van Mooy BAS et al. (2015). Resource allocation by the marine cyanobacterium Synechococcus WH8102 in response to different nutrient supply ratios. Limnol Oceanogr 60: 1634–1641.
Pahlow M, Oschlies A . (2009). Chain model of phytoplankton P, N and light colimitation. Mar Ecol Prog Ser 376: 69–83.
Rao NN, Gómez-García MR, Kornberg A . (2009). Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem 78: 605–647.
Rhee GY . (1978). Effects of N:P atomic ratios nitrate limitation on algal growth, cell composition, nitrate uptake. Limnol Oceanogr 23: 10–25.
Rhee G-Y . (1973). A continuous culture study of phosphate uptake, growth rate, and polyphosphate in Scenedesmus sp. J Phycol 9: 495–506.
Scanlan DJ, Ostrowski M, Mazard S, Dufresne A, Garczarek L, Hess WR et al. (2009). Ecological genomics of marine picocyanobacteria. Microbiol Mol Biol Rev 73: 249–299.
Schaechter M, MaalOe O, Kjeldgaard NO . (1958). Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. J Gen Microbiol 19: 592–606.
Shuter B . (1979). A model of physiological adaptation in unicellular algae. J Theor Biol 78: 519–552.
Sterner RW, Elser JJ . (2002) Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press: Princeton, NJ, USA.
Sterner RW, Elser JJ, Fee EJ, Guildford SJ, Chrzanowski TH . (1997). The light: nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am Nat 150: 663–684.
Teng Y-C, Primeau FW, Moore JK, Lomas MW, Martiny AC . (2014). Global-scale variations of the ratios of carbon to phosphorus in exported marine organic matter. Nat Geosci 7: 895–898.
Urabe J, Kyle M, Makino W, Yoshida T, Andersen T, Elser JJ . (2002). Reduced light increases herbivore production due to stoichiometric effects of light/nutrient balance. Ecology 83: 619–627.
Vadia S, Levin PA . (2015). Growth rate and cell size: a re-examination of the growth law. Curr Opin Microbiol 24: 96–103.
Van Mooy BAS, Rocap G, Fredricks HF, Evans CT, Devol AH . (2006). Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligotrophic marine environments. Proc Natl Acad Sci USA 103: 8607–8612.
Verspagen JMH, Van de Waal DB, Finke JF, Visser PM, Huisman J . (2014). Contrasting effects of rising CO2 on primary production and ecological stoichiometry at different nutrient levels. Ecol Lett 17: 951–960.
Waterbury J, Willey JM . (1988). Isolation and growth of marine planktonic Cyanobacteria. Methods Enzymol 167: 100–105.
Wingard LL, Miller SR, Sellker JML, Stenn E, Allen MM, Wood A M . (2002). Cyanophycin production in a phycoerythrin-containing marine Synechococcus strain of unusual phylogenetic affinity. Appl Environ Microbiol 68: 1772–1777.
Wyman M, Gregory RP, Carr NG . (1985). Novel role for phycoerythrin in a marine cyanobacterium, Synechococcus strain DC2. Science 230: 818–820.
Yeh SW, Ong LJ, Glazer AN . (1986). Role of phycoerythrin in marine picoplankton Synechococcus spp. Science 234: 1422–1423.
Zimmerman AE, Allison SD, Martiny AC . (2014). Phylogenetic constraints on elemental stoichiometry and resource allocation in heterotrophic marine bacteria. Environ Microbiol 16: 1398–1410.
Acknowledgements
Support for this research was provided by the UCI Chancellor’s ADVANCE Postdoctoral Program and the National Science Foundation Dimensions of Biodiversity (OCE-1046297) and Major Research Instrumentation programs (OCE-1126749). JAB acknowledges support of the MASTS pooling initiative (The Marine Alliance for Science and Technology for Scotland). MASTS is funded by the Scottish Funding Council (grant reference HR09011) and contributing institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Garcia, N., Bonachela, J. & Martiny, A. Interactions between growth-dependent changes in cell size, nutrient supply and cellular elemental stoichiometry of marine Synechococcus. ISME J 10, 2715–2724 (2016). https://doi.org/10.1038/ismej.2016.50
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2016.50
This article is cited by
-
Less variability when growing faster? Experimental assessment of the relationship of growth rate with functional traits of the marine diatom Phaeodactylum tricornutum
Hydrobiologia (2024)
-
Adaptive carbon export response to warming in the Sargasso Sea
Nature Communications (2022)
-
Global patterns and predictors of C:N:P in marine ecosystems
Communications Earth & Environment (2022)
-
Global patterns in marine organic matter stoichiometry driven by phytoplankton ecophysiology
Nature Geoscience (2022)
-
Growth rate-dependent coordination of catabolism and anabolism in the archaeon Methanococcus maripaludis under phosphate limitation
The ISME Journal (2022)