Abstract
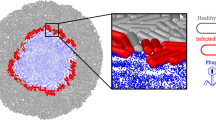
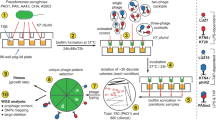
The activity of bacteriophages poses a major threat to bacterial survival. Upon infection, a temperate phage can either kill the host cell or be maintained as a prophage. In this state, the bacteria carrying the prophage is at risk of superinfection, where another phage injects its genetic material and competes for host cell resources. To avoid this, many phages have evolved mechanisms that alter the bacteria and make it resistant to phage superinfection. The mechanisms underlying these phentoypic conversions and the fitness consequences for the host are poorly understood, and systematic studies of superinfection exclusion mechanisms are lacking. In this study, we examined a wide range of Pseudomonas aeruginosa phages and found that they mediate superinfection exclusion through a variety of mechanisms, some of which affected the type IV pilus and O-antigen, and others that functioned inside the cell. The strongest resistance mechanism was a surface modification that we showed is cost-free for the bacterial host in a natural soil environment and in a Caenorhabditis. elegans infection model. This study represents the first systematic approach to address how a population of prophages influences phage resistance and bacterial behavior in P. aeruginosa.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ackermann H-W, Cartier C, Slopek S, Vieu JF . (1988). Morphology of Pseudomonas aeruginosa typing phages of the Lindberg set. Ann Inst Pasteur Virol 139: 389–404.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ . (1990). Basic local alignment search tool. J Mol Biol 215: 403–410.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA et al. (2008). The RAST Server: rapid annotations using subsystems technology. BMC genomics 9: 75–99.
Bergh O, Børsheim KY, Bratbak G, Heldal M . (1989). High abundance of viruses found in aquatic environments. Nature 340: 467–468.
Bondy-Denomy J, Davidson AR . (2014). When a virus is not a parasite: the beneficial effects of prophages on bacterial fitness. J Microbiol (Seoul, Korea) 52: 235–242.
Bondy-Denomy J, Pawluk A, Maxwell KL, Davidson AR . (2013). Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature 493: 429–432.
Boulanger P, Letellier L . (1988). Characterization of ion channels involved in the penetration of phage T4 DNA into Escherichia coli cells. J Biol Chem 263: 9767–9775.
Boulanger P, Letellier L . (1992). Ion channels are likely to be involved in the two steps of phage T5 DNA penetration into Escherichia coli cells. J Biol Chem 267: 3168–3172.
Bourkal’tseva MV, Krylov SV, Kropinski AM, Pleteneva EA, Shaburova OV, Krylov VN . (2011). Bacteriophage phi297, a new species of Pseudomonas aeruginosa temperate phages with a mosaic genome: potential use in phage therapy. Russ J Genet 47: 794–798.
Bradley DE . (1972). Evidence for the retraction of Pseudomonas aeruginosa RNA phage pili. Biochem Biophys Res Commun 47: 142–149.
Bradley DE . (1973). Basic characterization of a Pseudomonas aeruginosa pilus-dependent bacteriophage with a long noncontractile tail. J Virol 12: 1139–1148.
Buckling A, Brockhurst M . (2012). Bacteria–virus coevolution. Adv Exp Med Biol 751: 347–370.
Burrows LL . (2012). Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu Rev Microbiol 66: 493–520.
Canchaya C, Proux C, Fournous G, Bruttin A, Brüssow H . (2003). Prophage genomics. Microbiol Mol Biol Rev 67: 238–276.
Cazares A, Mendoza-Hernández G, Guarneros G . (2014). Core and accessory genome architecture in a group of Pseudomonas aeruginosa Mu-like phages. BMC Genomics 15: 1146.
Chung I-Y, Cho Y-H . (2012). Complete genome sequences of two Pseudomonas aeruginosa temperate phages, MP29 and MP42, which lack the phage–host CRISPR interaction. J Virol 86: 8336.
Chung I-Y, Jang HJ, Bae HW, Cho Y-H . (2014). A phage protein that inhibits the bacterial ATPase required for type IV pilus assembly. Proc Natl Acad Sci USA 111: 11503–11508.
Cumby N, Edwards AM, Davidson AR, Maxwell KL . (2012). The bacteriophage HK97 gp15 moron element encodes a novel superinfection exclusion protein. J Bacteriol 194: 5012–5019.
Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H et al. (1998). Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun 66: 43–51.
Heo Y-J, Chung I-Y, Choi KB, Lau GW, Cho Y-H . (2007). Genome sequence comparison and superinfection between two related Pseudomonas aeruginosa phages, D3112 and MP22. Microbiology (Reading, England) 153 (Part 9): 2885–2895.
Huang Y, Niu B, Gao Y, Fu L, Li W . (2010). CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26: 680–682.
James CE, Fothergill JL, Kalwij H, Hall AJ, Cottell J, Brockhurst MA et al. (2012). Differential infection properties of three inducible prophages from an epidemic strain of Pseudomonas aeruginosa. BMC Microbiol 12: 216.
Jenkins ATA, Buckling A, McGhee M, ffrench-Constant RH . (2005). Surface plasmon resonance shows that type IV pili are important in surface attachment by Pseudomonas aeruginosa. J R Soc Interface 2: 255–259.
Kropinski AM . (2000). Sequence of the genome of the temperate, serotype-converting Pseudomonas aeruginosa bacteriophage D3. J Bacteriol 182: 6066–6074.
Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G et al. (2006). An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci USA 103: 2833–2838.
Newton GJ, Daniels C, Burrows LL, Kropinski AM, Clarke AJ, Lam JS . (2001). Three-component-mediated serotype conversion in Pseudomonas aeruginosa by bacteriophage D3. Mol Microbiol 39: 1237–1247.
Parkins MD, Glezerson BA, Sibley CD, Sibley KA, Duong J, Purighalla S et al. (2014). Twenty-five-year outbreak of Pseudomonas aeruginosa infecting individuals with cystic fibrosis: identification of the prairie epidemic strain. J Clin Microbiol 52: 1127–1135.
Pawluk A, Bondy-Denomy J, Cheung VHW, Maxwell KL, Davidson AR . (2014). A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR-Cas system of Pseudomonas aeruginosa. mBio 5: e00896.
Pell LG, Kanelis V, Donaldson LW, Howell PL, Davidson AR . (2009). The phage lambda major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc Natl Acad Sci USA 106: 4160–4165.
Salmon KA, Freedman O, Ritchings BW, DuBow MS . (2000). Characterization of the lysogenic repressor (c) gene of the Pseudomonas aeruginosa transposable bacteriophage D3112. Virology 272: 85–97.
Samson JE, Magadán AH, Sabri M, Moineau S . (2013). Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol 11: 675–687.
Söding J . (2005). Protein homology detection by HMM-HMM comparison. Bioinformatics (Oxford, England) 21: 951–960.
Söding J, Biegert A, Lupas AN . (2005). The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 33: W244–W248.
Stayrook S, Jaru-Ampornpan P, Ni J, Hochschild A, Lewis M . (2008). Crystal structure of the lambda repressor and a model for pairwise cooperative operator binding. Nature 452: 1022–1025.
Sun X, Göhler A, Heller KJ, Neve H . (2006). The ltp gene of temperate Streptococcus thermophilus phage TP-J34 confers superinfection exclusion to Streptococcus thermophilus and Lactococcus lactis. Virology 350: 146–157.
Susskind MM, Wright A, Botstein D . (1974). Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. IV. Genetics and physiology of sieB exclusion. Virology 2: 367–384.
Suttle CA . (2005). Viruses in the sea. Nature 437: 356–361.
Suttle CA . (2007). Marine viruses—major players in the global ecosystem. Nat Rev Microbiol 5: 801–812.
Tamura K, Nei M . (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10: 512–526.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S . (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739.
Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM . (1999). Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA 96: 2408–2413.
Uc-Mass A, Loeza EJ, de la Garza M, Guarneros G, Hernández-Sánchez J, Kameyama L . (2004). An orthologue of the cor gene is involved in the exclusion of temperate lambdoid phages. Evidence that Cor inactivates FhuA receptor functions. Virology 329: 425–433.
Waldor MK, Friedman DI . (2005). Phage regulatory circuits and virulence gene expression. Curr Opin Microbiol 8: 459–465.
Wang PW, Chu L, Guttman DS . (2004). Complete sequence and evolutionary genomic analysis of the Pseudomonas aeruginosa transposable bacteriophage D3112. J Bacteriol 186: 400–410.
Westra ER, van Houte S, Oyesiku-Blakemore S, Makin B, Broniewski JM, Best A et al. (2015). Parasite exposure drives selective evolution of constitutive versus inducible defense. Curr Biol 25: 1043–1049.
Winstanley C, Langille MGI, Fothergill JL, Kukavica-Ibrulj I, Paradis-Bleau C, Sanschagrin F et al. (2009). Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool Epidemic Strain of Pseudomonas aeruginosa. Genome Res 19: 12–23.
Wommack KE, Colwell RR . (2000). Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64: 69–114.
Zegans ME, Wagner JC, Cady KC, Murphy DM, Hammond JH, O'Toole GA . (2009). Interaction between bacteriophage DMS3 and host CRISPR region inhibits group behaviors of Pseudomonas aeruginosa. J Bacteriol 191: 210–219.
Acknowledgements
This work was supported by operating grants from the Canadian Institutes of Health Research to KLM (MOP-136845) and ARD (XNE-86943). JB-D was supported by a CIHR Canada Graduate Scholarship Doctoral Award and an Ontario Graduate Scholarship. DSG is supported by NSERC. ERW was supported by NERC and Wellcome Trust. AB acknowledges NERC, AXA, BBSRC and the Royal Society for funding. We thank Andrew Spence, Kathy Shire, Steven Doyle and Battista Calvieri for advice and technical assistance. We also thank Derrick Fouts at the J Craig Venter Institute for phage sequencing (NIH U54 AI84844-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Bondy-Denomy, J., Qian, J., Westra, E. et al. Prophages mediate defense against phage infection through diverse mechanisms. ISME J 10, 2854–2866 (2016). https://doi.org/10.1038/ismej.2016.79
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2016.79
This article is cited by
-
Genetic diversity of RNA viruses infecting invertebrate pests of rice
Science China Life Sciences (2024)
-
Insights into Superinfection Immunity Regulation of Xanthomonas axonopodis Filamentous Bacteriophage cf
Current Microbiology (2024)
-
Soil viral diversity, ecology and climate change
Nature Reviews Microbiology (2023)
-
Genome analysis of triple phages that curtails MDR E. coli with ML based host receptor prediction and its evaluation
Scientific Reports (2023)
-
Life strategies for Aminicenantia in subseafloor oceanic crust
The ISME Journal (2023)