Abstract
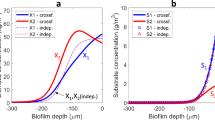
Metabolic interactions between populations can influence patterns of spatial organization and diversity within microbial communities. Cross-feeding is one type of metabolic interaction that is pervasive within microbial communities, where one genotype consumes a resource into a metabolite while another genotype then consumes the metabolite. A typical feature of cross-feeding is that the metabolite may impose toxicity if it accumulates to sufficient concentrations. However, little is known about the effect of metabolite toxicity on spatial organization and local diversity within microbial communities. We addressed this knowledge gap by experimentally varying the toxicity of a single cross-fed metabolite and measuring the consequences on a synthetic microbial cross-feeding community. Our results demonstrate that metabolite toxicity slows demixing and thus slows local diversity loss of the metabolite-producing population. Using mathematical modeling, we show that this is because toxicity slows growth, which enables more cells to emigrate from the founding region and contribute towards population expansion. Our results show that metabolite toxicity is an important factor affecting local diversity within microbial communities and that spatial organization can be affected by non-intuitive mechanisms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Battin TJ, Sloan WT, Kjelleberg S, Daims H, Head IM, Curtis TP et al. (2007). Microbial landscapes: new paths to biofilm research. Nat Rev Microbiol 5: 76–81.
Bull JJ, Harcombe WR . (2009). Population dynamics constrain the cooperative evolution of cross-feeding. PLoS One 4: e4115.
Davey ME, O’Toole GA . (2000). Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64: 847–867.
Dolinšek J, Goldschmidt F, Johnson DR . (2016). Synthetic microbial ecology and the dynamic interplay between microbial genotypes. FEMS Microbiol Rev 40: 961–979.
Estrela S, Gudelj I . (2010). Evolution of cooperative cross-feeding could be less challenging than originally thought. PLoS One 5: e14121.
Estrela S, Trisos CH, Brown SP . (2012). From metabolism to ecology: cross-feeding interactions shape the balance between polymicrobial conflict and mutualism. Am Nat 180: 566–576.
Excoffier L, Foll M, Petit RJ . (2009). Genetic consequences of range expansions. Annu Rev Ecol Evol Syst 40: 481–501.
Ferreira TA, Blackman AV, Oyrer J, Jayabal S, Chung AJ, Watt AJ et al. (2014). Neuronal morphometry directly from bitmap images. Nat Methods 11: 982–984.
Goldschmidt F, Regoes RR, Johnson DR . (2017). Successive range expansion promotes diversity and accelerates evolution in spatially structured microbial populations. ISME J 11: 2112–2123.
Gralka M, Stiewe F, Farrell F, Möbius W, Waclaw B, Hallatschek O . (2016). Allele surfing promotes microbial adaptation from standing variation. Ecol Lett 19: 889–898.
Hallatschek O, Hersen P, Ramanathan S, Nelson DR . (2007). Genetic drift at expanding frontiers promotes gene segregation. PNAS 104: 19926–19930.
Harcombe WR, Riehl WJ, Dukovski I, Granger BR, Betts A, Lang AH et al. (2014). Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep 7: 1104–1115.
Hodgson E . (2004) A Textbook of Modern Toxicology. John Wiley & Sons, Inc.: Hoboken, NJ, USA.
Johnson DR, Goldschmidt F, Lilja EE, Ackermann M . (2012). Metabolic specialization and the assembly of microbial communities. ISME J 11: 1985–1991.
Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF . (2008). Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci USA 105: 18188–18193.
Korolev KS, Xavier JB, Nelson DR, Foster KR . (2011). A quantitative test of population genetics using spatiogenetic patterns in bacterial colonies. Am Nat 178: 538–552.
Lilja EE, Johnson DR . (2016). Segregating metabolic processes into different microbial cells accelerates the consumption of inhibitory substrates. ISME J 10: 1568–1578.
Lindemann SR, Bernstein HC, Song HS, Frederickson JK, Fields MW, Shou W et al. (2016). Engineering microbial consortia for controllable outputs. ISME J 10: 2077–2084.
Martienssen M, Schöps R . (1999). Population dynamics of denitrifying bacteria in a model biocommunity. Water Res 33: 639–646.
McInerney MJ, Sieber JR, Gunsalus RP . (2009). Syntrophy in anaerobic global carbon cycles. Curr Opin Biotechnol 20: 623–632.
Minoia M, Gaillard M, Reinhard F, Stojanov M, Sentchilo V, van der Meer JR . (2008). Stochasticity and bistability in horizontal transfer control of a genomic island in Pseudomonas. Proc Natl Acad Sci U S A 105: 20792–20797.
Mitri S, Clarke E, Foster KR . (2015). Resource limitation drives spatial organization in microbial groups. ISME J, 1–12.
Mitri S, Xavier JB, Foster KR . (2011). Social evolution in multispecies biofilms. Proc Natl Acad Sci USA 108: 10839–10846.
Momeni B, Waite AJ, Shou W . (2013). Spatial self-organization favors heterotypic cooperation over cheating. Elife 2: e00960.
Morris BEL, Henneberger R, Huber H, Moissl-Eichinger C . (2013). Microbial syntrophy: Interaction for the common good. FEMS Microbiol Rev 37: 384–406.
Müller MJI, Neugeboren BI, Nelson DR, Murray AW . (2014). Genetic drift opposes mutualism during spatial population expansion. Proc Natl Acad Sci USA 111: 1037–1042.
Nadell CD, Foster KR, Xavier JB . (2010). Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput Biol 6: e1000716.
Nichols DS, Olley J, Garda H, Brenner RR, McMeekin TA . (2000). Effect of temperature and salinity stress on growth and lipid composition of Shewanella gelidimarina. Appl Environ Microbiol 66: 2422–2429.
Pfeiffer T, Bonhoeffer S . (2004). Evolution of cross-feeding in microbial populations. Am Nat 163: E126–E135.
R Core Team. (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
Rainey PB, Buckling A, Kassen R, Travisano M . (2000). The emergence and maintenance of diversity: insights from experimental bacterial populations. Trends Ecol Evol 15: 243–247.
Schink B . (1997). Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev 61: 262–280.
Schink B . (2002). Synergistic interactions in the microbial world. Antonie Van Leeuwenhoek 81: 257–261.
Schneider CA, Rasband WS, Eliceiri KW . (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675.
Sijbesma WFH, Almeida JS, Reis MAM, Santos H . (1996). Uncoupling effect of nitrite during denitrification by Pseudomonas fluorescens: an in vivo 31P-NMR study. Biotechnol Bioeng 52: 176–182.
de Souza ML, Newcombe D, Alvey S, Crowley DE, Hay A, Sadowsky MJ et al. (1998). Molecular basis of a bacterial consortium: interspecies catabolism of atrazine. Appl Environ Microbiol 64: 178–184.
West Sa, Diggle SP, Buckling A, Gardner A, Griffin AS . (2007). The social lives of microbes. Annu Rev Ecol Evol Syst 38: 53–77.
Zhou Y, Oehmen A, Lim M, Vadivelu V, Ng WJ . (2011). The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res 45: 4672–4682.
Zumft WG . (1997). Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61: 533–616.
Zumft WG . (1993). The biological role of nitric oxide in bacteria. Arch Microbiol 160: 253–264.
Acknowledgements
We acknowledge Martin Ackermann, Benedict Borer, Sara Mitri, Jonas Schulter and Simon Norrelykke for useful discussions and Simon van Vliet for helpful comments on a preliminary version of the manuscript. We also thank three anonymous reviewers for significantly improving the quality and clarity of this manuscript. This work was supported by grants from the Swiss National Science Foundation (31003A_132905, 31003A_149304) and SystemsX.ch, The Swiss Initiative in Systems Biology (MicroScapesX.ch).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Goldschmidt, F., Regoes, R. & Johnson, D. Metabolite toxicity slows local diversity loss during expansion of a microbial cross-feeding community. ISME J 12, 136–144 (2018). https://doi.org/10.1038/ismej.2017.147
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/ismej.2017.147
This article is cited by
-
Disentangling the feedback loops driving spatial patterning in microbial communities
npj Biofilms and Microbiomes (2025)
-
Evaporation-induced hydrodynamics control plasmid transfer during surface-associated microbial growth
npj Biofilms and Microbiomes (2023)
-
Timing of antibiotic administration determines the spread of plasmid-encoded antibiotic resistance during microbial range expansion
Nature Communications (2023)
-
Rare and localized events stabilize microbial community composition and patterns of spatial self-organization in a fluctuating environment
The ISME Journal (2022)
-
Initial community composition determines the long-term dynamics of a microbial cross-feeding interaction by modulating niche availability
ISME Communications (2022)