Abstract
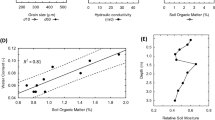
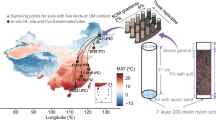
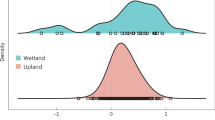
The priming effect in soil is proposed to be generated by two distinct mechanisms: ‘stoichiometric decomposition’ and/or ‘nutrient mining’ theories. Each mechanism has its own dynamics, involves its own microbial actors, and targets different soil organic matter (SOM) pools. The present study aims to evaluate how climatic parameters drive the intensity of each priming effect generation mechanism via the modification of soil microbial and physicochemical properties. Soils were sampled in the center of Madagascar, along climatic gradients designed to distinguish temperature from rainfall effects. Abiotic and biotic soil descriptors were characterized including bacterial and fungal phylogenetic composition. Potential organic matter mineralization and PE were assessed 7 and 42 days after the beginning of incubation with 13C-enriched wheat straw. Both priming mechanisms were mainly driven by the mean annual temperature but in opposite directions. The priming effect generated by stoichiometric decomposition was fostered under colder climates, because of soil enrichment in less developed organic matter, as well as in fast-growing populations. Conversely, the priming effect generated by nutrient mining was enhanced under warmer climates, probably because of the lack of competition between slow-growing populations mining SOM and fast-growing populations for the energy-rich residue entering the soil. Our study leads to hypotheses about the consequences of climate change on both PE generation mechanisms and associated consequences on soil carbon sequestration.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Amer F, Bouldin DR, Black CA, Duke FR . (1955). Characterization of soil phosphorus by anion exchange resin adsorption and P32-equilibration. Plant Soil 6: 391–408.
Becquer T, Pétard J, Duwig C, Bourdon E, Moreau R, Herbillon AJ . (2001). Mineralogical, chemical and charge properties of Geric Ferralsols from New Caledonia. Geoderma 103: 291–306.
Bernard L, Chapuis-Lardy L, Razafimbelo T, Razafindrakoto M, Pablo A-L, Legname E et al. (2012). Endogeic earthworms shape bacterial functional communities and affect organic matter mineralization in a tropical soil. ISME J 6: 213–222.
Bernard L, Maron PA, Mougel C, Nowak V, Lévêque J, Marol C et al. (2009). Contamination of soil by copper affects the dynamics, diversity, and activity of soil bacterial communities involved in wheat decomposition and carbon storage. Appl Environ Microbiol 75: 7565–7569.
Bernard L, Mougel C, Maron P-A, Nowak V, Lévêque J, Henault C et al. (2007). Dynamics and identification of soil microbial populations actively assimilating carbon from 13C-labelled wheat residue as estimated by DNA- and RNA-SIP techniques. Environ Microbiol 9: 752–764.
Bingeman CW, Varner JE, Martin WP . (1953). The effect of the addition of organic materials on the decomposition of an organic soil. Soil Sci Soc Am J 17: 34–38.
Blagodatskaya E, Khomyakov N, Myachina O, Bogomolova I, Blagodatsky S, Kuzyakov Y . (2014). Microbial interactions affect sources of priming induced by cellulose. Soil Biol Biochem 74: 39–49.
Bonkowski M, Griffiths BS, Scrimgeour C . (2000). Substrate heterogeneity and microfauna in soil organic ‘hotspots’ as determinants of nitrogen capture and growth of rye-grass. Appl Soil Ecol 14: 37–53.
Calvet R, Barriuso E, Dubus IG . (2007). Application of two surface complexation models to the adsorption of weak organic acids by soil: an additive approach. Eur J Soil Science 58: 609–624.
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X et al. (2014). Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Change Biol 20: 2356–2367.
Ciesielski H, Sterckeman T, Santerne M, Willery JP . (1997). Determination of cation exchange capacity and exchangeable cations in soils by means of cobalt hexamine trichloride. Effects of experimental conditions. Agronomie 17: 1–7.
Davidson EA, Janssens IA . (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440: 165–173.
Davis KER, Sangwan P, Janssen PH . (2011). Acidobacteria, Rubrobacteridae and Chloroflexi are abundant among very slow-growing and mini-colony-forming soil bacteria. Environ Microbiol 13: 798–805.
de Boer W, Folman LB, Summerbell RC, Boddy L . (2005). Living in a fungal world: impact of fungi on soil bacterial niche development. Fems Microbiol Rev 29: 795–811.
de Junet A, Basile-Doelsch I, Borschneck D, Masion A, Legros S, Christine Marole C et al. (2013). Characterisation of organic matter from organo-mineral complexes in an Andosol from Reunion Island. J Anal Appl Pyrolysis 99: 92–100.
Dequiedt S, Saby NPA, Lelievre M, Jolivet C, Thioulouse J, Toutain B et al. (2011). Biogeographical patterns of soil molecular microbial biomass as influenced by soil characteristics and management. Glob Ecol Biogeogr 20: 641–652.
Derrien D, Plain C, Courty P-E, Gelhaye L, Moerdijk-Poortvliet TCW, Thomas F et al. (2014). Does the addition of labile substrate destabilise old soil organic matter? Soil Biol Biochem 76: 149–160.
Eilers KG, Lauber CL, Knight R, Fierer N . (2010). Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol Biochem 42: 896–903.
Ekelund F, Rønn R . (1994). Notes on protozoa in agricultural soil with emphasis on heterotrophic flagellates and naked amoebae and their ecology. FEMS Microbiol Rev 15: 321–353.
Eliasson PE, McMurtrie RE, Pepper DA, Strömgren M, Linder S, Ågren GI . (2005). The response of heterotrophic CO2 flux to soil warming. Glob Change Biol 11: 167–181.
Fierer N, Bradford MA, Jackson RB . (2007). Toward an ecological classification of soil bacteria. Ecology 88: 1354–1364.
Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R . (2012). Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6: 1007–1017.
Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC . (2009). Global patterns in belowground communities. Ecol Lett 12: 1238–1249.
Foesel BU, Nägele V, Naether A, Wüst PK, Weinert J, Bonkowski M et al. (2014). Determinants of Acidobacteria activity inferred from the relative abundances of 16 S rRNA transcripts in German grassland and forest soils. Environ Microbiol 16: 658–675.
Foesel BU, Rohde M, Overmann J . (2013). Blastocatella fastidiosa gen. nov., sp. nov., isolated from semiarid savanna soil – The first described species of Acidobacteria subdivision 4. Syst Appl Microbiol 36: 82–89.
Fontaine S, Mariotti A, Abbadie L . (2003). The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35: 837–843.
Frøseth RB, Bleken MA . (2015). Effect of low temperature and soil type on the decomposition rate of soil organic carbon and clover leaves, and related priming effect. Soil Biol Biochem 80: 156–166.
Gao Q, Schwartz MW, Zhu W, Wan Y, Qin X, Ma X et al. (2016). Changes in Global Grassland Productivity during 1982 to 2011 Attributable to Climatic Factors. Remote Sens 8: 384.
Geyer KM, Kyker-Snowman E, Grandy AS, Frey SD . (2016). Microbial carbon use efficiency: accounting for population, community, and ecosystem-scale controls over the fate of metabolized organic matter. Biogeochem 127: 173–188.
Griffiths BS . (1994). Microbial-feeding nematodes and protozoa in soil: Their effects on microbial activity and nitrogen mineralization in decomposition hotspots and the rhizosphere. Plant Soil 164: 25–33.
Heimann M, Reichstein M . (2008). Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451: 289–292.
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A . (2005). Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25: 1965–1978.
Hunt JF, Ohno T, He Z, Honeycutt CW, Dail CB . (2007). Inhibition of phosphorus sorption to goethite, gibbsite, and kaolin by fresh and decomposed organic matter. Biol Fertil Soils 44: 277–288.
Imberger KT, Chiu C-Y . (2001). Spatial changes of soil fungal and bacterial biomass from a sub-alpine coniferous forest to grassland in a humid, sub-tropical region. Biol Fertil Soils 33: 105–110.
Jackson ML . (1958) Soil Chemical Analysis. Prentice Hall Inc: Englewood Cliffs, NJ, USA.
Janssen PH . (2006). Identifying the dominant soil bacterial taxa in libraries of 16 S rRNA and 16 S rRNA genes. Appl Environ Microbiol 72: 1719–1728.
Johnson NC, Wedin DA . (1997). Soil carbon, nutrients, and mycorrhizae during conversion of dry tropical forest to grassland. Ecol Appl 7: 171–182.
Jorquera MA, Hernández MT, Rengel Z, Marschner P, Mora M, de la L . (2008). Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol Fertil Soils 44: 1025–1034.
Kedi B, Abadie J, Sei J, Quiquampoix H, Staunton S . (2013). Diversity of adsorption affinity and catalytic activity of fungal phosphatases adsorbed on some tropical soils. Soil Biol Biochem 56: 13–20.
Klappenbach JA, Dunbar JM, Schmidt TM . (2000). rRNA Operon Copy Number Reflects Ecological Strategies of Bacteria. Appl Environ Microbiol 66: 1328–1333.
Kouno K, Tuchiya Y, Ando T . (1995). Measurement of soil microbial biomass phosphorus by an anion-exchange membrane method. Soil Biol Biochem 27: 1353–1357.
Kuzyakov Y, Friedel J, Stahr K . (2000). Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32: 1485–1498.
Lienhard P, Tivet F, Chabanne A, Dequiedt S, Lelièvre M, Sayphoummie S et al. (2012). No-till and cover crops shift soil microbial abundance and diversity in Laos tropical grasslands. Agron Sustain Dev 33: 375–384.
Luo Y, Wan S, Hui D, Wallace LL . (2001). Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413: 622–625.
Maron P-A, Mougel C, Ranjard L . (2011). Soil microbial diversity: Methodological strategy, spatial overview and functional interest. C R Biol 334: 403–411.
Marstorp H, Guan X, Gong P . (2000). Relationship between dsDNA, chloroform labile C and ergosterol in soils of different organic matter contents and pH. Soil Biol Biochem 32: 879–882.
Moorhead DL, Sinsabaugh RL . (2006). A theoretical model of litter decay and microbial interaction. Ecol Monogr 76: 151–174.
Mulvaney ML . (1986) Nitrogen—Inorganic forms. In: Klute A (ed.) Methods of Soil Analysis Part 3. Chemical Methods. American Society of Agronomy: Madison, WI, USA, p 1130.
Murphy CJ, Baggs EM, Morley N, Wall DP, Paterson E . (2015). Rhizosphere priming can promote mobilisation of N-rich compounds from soil organic matter. Soil Biol Biochem 81: 236–243.
Neelin JD, Münnich M, Su H, Meyerson JE, Holloway CE . (2006). Tropical drying trends in global warming models and observations. PNAS 103: 6110–6115.
Pansu M, Gautheyrou J . (2007) Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods. Springer Science & Business Media: Berlin, Heidelberg, The Netherlands.
Pascault N, Ranjard L, Kaisermann A, Bachar D, Christen R, Terrat S et al. (2013). Stimulation of different functional groups of bacteria by various plant residues as a driver of soil priming effect. Ecosystems 16: 810–822.
Pavinato PS, Dao TH, Rosolem CA . (2010). Tillage and phosphorus management effects on enzyme-labile bioactive phosphorus availability in Cerrado Oxisols. Geoderma 156: 207–215.
Plassart P, Terrat S, Thomson B, Griffiths R, Dequiedt S, Lelievre M et al. (2012). Evaluation of the ISO standard 11063 DNA extraction procedure for assessing soil microbial abundance and community structure. PLoS One 7: e44279.
Ramarosona VH, Becquer T, Sá SO, Razafimahatratra H, Larvy Delarivière J, Blavet D et al. (2017). Mineralogical analysis of ferralitic soils in Madagascar using NIR spectroscopy. Catena. Available at: https://doi.org/10.1016/j.catena.2017.07.016.
Ranjard L, Richaume A . (2001). Quantitative and qualitative microscale distribution of bacteria in soil. Res Microbiol 152: 707–716.
Rustad L, Campbell J, Marion G, Norby R, Mitchell M, Hartley A et al. (2001). A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126: 543–562.
Saggar S, Parshotam A, Sparling GP, Feltham CW, Hart PBS . (1996). 14C-labelled ryegrass turnover and residence times in soils varying in clay content and mineralogy. Soil Biol Biochem 28: 1677–1686.
Schaefer CEGR, Fabris JD, Ker JC . (2008). Minerals in the clay fraction of Brazilian Latosols (Oxisols): a review. Clay Miner 43: 137–154.
Tardy V, Spor A, Mathieu O, Lévèque J, Terrat S, Plassart P et al. (2015). Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol Biochem 90: 204–213.
Terrat S, Christen R, Dequiedt S, Lelièvre M, Nowak V, Regnier T et al. (2012). Molecular biomass and MetaTaxogenomic assessment of soil microbial communities as influenced by soil DNA extraction procedure. Microb Biotechnol 5: 135–141.
van Breemen N, Mulder J, Driscoll CT . (1983). Acidification and alkalinization of soils. Plant Soil 75: 283–308.
Wardle DA . (2005) How plant communities influence decomposer communities. In: Bardgett R, Usher M, Hopkins D (eds.) Biological Diversity and Function in Soils (Ecological Reviews). Cambridge University Press: Cambridge, UK, pp. 119–138.
Widmer F, Rasche F, Hartmann M, Fliessbach A . (2006). Community structures and substrate utilization of bacteria in soils from organic and conventional farming systems of the DOK long-term field experiment. Appl Soil Ecol 33: 294–307.
Will C, Thürmer A, Wollherr A, Nacke H, Herold N, Schrumpf M et al. (2010). Horizon-specific bacterial community composition of german grassland soils, as revealed by pyrosequencing-based analysis of 16 S rRNA genes. Appl Environ Microbiol 76: 6751–6759.
Acknowledgements
The present study was funded by the French Foundation for Research on Biodiversity (FRB- AAP-SCEN-2013 II – CAMMiSolE project). The first author was awarded with a 5-month mobility research grant from Madagascar to France, funded by the Agropolis Foundation (AAP Open Science- CARIM project). We would like to thank Modeste Rakotondramanana (IRD) and Fidy Raharison (LRI) for their help with field sampling and Marie-Paule Razafimanantsoa (LRI) for her coaching during laboratory analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Razanamalala, K., Razafimbelo, T., Maron, PA. et al. Soil microbial diversity drives the priming effect along climate gradients: a case study in Madagascar. ISME J 12, 451–462 (2018). https://doi.org/10.1038/ismej.2017.178
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2017.178
This article is cited by
-
Distinct patterns of soil bacterial and fungal communities in the treeline ecotone
Soil Ecology Letters (2025)
-
The fungal community assembly was governed by deterministic selection during priming effects induced by residue addition
Plant and Soil (2025)
-
Shifts in microbial mechanism regulate carbon priming effect under two simulated root exudate and nitrogen addition in coniferous and broad-leaved forest soils
Plant and Soil (2025)
-
Adding glucose at a uniform amount or according to soil organic carbon content does not cause significant differences in soil priming effects across a broad grassland transect
Soil Ecology Letters (2025)
-
Distinctive mechanisms of soil priming in different stages and its response to nitrogen addition along a temperate forest elevation gradient
Soil Ecology Letters (2025)