Abstract
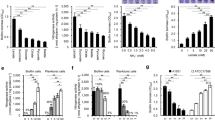
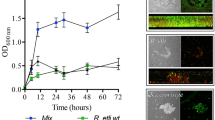
The multicellular communities of microorganisms known as biofilms are of high significance in agricultural setting, yet it is largely unknown about the biofilm formed by nitrogen-fixing bacteria. Here we report the biofilm formation by Pseudomonas stutzeri A1501, a free-living rhizospheric bacterium, capable of fixing nitrogen under microaerobic and nitrogen-limiting conditions. P. stutzeri A1501 tended to form biofilm in minimal media, especially under nitrogen depletion condition. Under such growth condition, the biofilms formed at the air–liquid interface (termed as pellicles) and the colony biofilms on agar plates exhibited nitrogenase activity in air. The two kinds of biofilms both contained large ovoid shape ‘cells’ that were multiple living bacteria embedded in a sac of extracellular polymeric substances (EPSs). We proposed to name such large ‘cells’ as A1501 cyst. Our results suggest that the EPS, especially exopolysaccharides enabled the encased bacteria to fix nitrogen while grown under aerobic condition. The formation of A1501 cysts was reversible in response to the changes of carbon or nitrogen source status. A1501 cyst formation depended on nitrogen-limiting signaling and the presence of sufficient carbon sources, yet was independent of an active nitrogenase. The pellicles formed by Azospirillum brasilense, another free-living nitrogen-fixing rhizobacterium, which also exhibited nitrogenase activity and contained the large EPS-encapsuled A1501 cyst-like ‘cells’. Our data imply that free-living nitrogen-fixing bacteria could convert the easy-used carbon sources to exopolysaccharides in order to enable nitrogen fixation in a natural aerobic environment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Assmus B, Hutzler P, Kirchhof G, Amann R, Lawrence JR, Hartmann A . (1995). In situ localization of Azospirillum brasilense in the rhizosphere of wheat with fluorescently labeled, rRNA-targeted oligonucleotide probes and scanning confocal laser microscopy. Appl Environ Microbiol 61: 1013–1019.
Baldani JI, Reis VM, Videira SS, Boddey LH, Baldani VL . (2014). The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384: 19.
Berg RH, Tyler ME, Novick NJ, Vasil V, Vasil IK . (1980). Biology of azospirillum-sugarcane association: enhancement of nitrogenase activity. Appl Environ Microbiol 39: 642–649.
Berleman JE, Bauer CE . (2004). Characterization of cyst cell formation in the purple photosynthetic bacterium Rhodospirillum centenum. Microbiology 150: 383–390.
Bible AN, Khalsa-Moyers GK, Mukherjee T, Green CS, Mishra P, Purcell A et al. (2015). Metabolic adaptations of Azospirillum brasilense to oxygen stress by cell-to-cell clumping and flocculation. Appl Environ Microbiol 81: 8346–8357.
Bleakley BH, Gaskins MH, Hubbell DH, Zam SG . (1988). Floc formation by Azospirillum lipoferum grown on poly-β-hydroxybutyrate. Appl Environ Microbiol 54: 2986–2995.
Branda SS, Vik S, Friedman L, Kolter R . (2005). Biofilms: the matrix revisited. Trends Microbiol 13: 20–26.
Burdman S, Okon Y, Jurkevitch E . (2000). Surface characteristics of Azospirillum brasilense in relation to cell aggregation and attachment to plant roots. Critical Rev Microbiol 26: 91–110.
Burris RH, Roberts GP . (1993). Biological nitrogen fixation. Annu Rev Nutr 13: 317–335.
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM . (1995). Microbial biofilms. Annu Rev Microbiol 49: 711–745.
DeLoughery A, Dengler V, Chai Y, Losick R . (2015). Biofilm formation by Bacillus subtilis requires an endoribonuclease-containing multisubunit complex that controls mRNA levels for the matrix gene repressor SinR. Mol Microbiol. 99: 425–437.
Desnoues N, Lin M, Guo X, Ma L, Carreno-Lopez R, Elmerich C . (2003). Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology 149: 2251–2262.
Dixon R, Kahn D . (2004). Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2: 621–631.
Drenkard E, Ausubel FM . (2002). Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416: 740–743.
Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F . (1951). A colorimetric method for the determination of sugars. Nature 168: 167.
Flemming HC, Wingender J . (2010). The biofilm matrix. Nat Rev Microbiol 8: 623–633.
Galimand M, Perroud B, Delorme F, Paquelin A, Vieille C, Bozouklian H et al. (1989). Identification of DNA regions homologous to nitrogen fixation genes nifE, nifUS and fixABC in Azospirillum brasilense Sp7. J Gen Microbiol 135: 1047–1059.
Hall-Stoodley L, Stoodley P . (2005). Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol 13: 7–10.
Hartmann A, Fu H, Burris RH . (1986). Regulation of nitrogenase activity by ammonium chloride in Azospirillum spp. J Bacteriol 165: 864–870.
Hebbar KP, Gueniot B, Heyraud A, Colinmorel P, Heulin T, Balandreau J et al. (1992). Characterization of exopolysaccharides produced by rhizobacteria. Appl Microbiol Biot 38: 248–253.
Herath H, Menikdiwela K, Igalavithana A, Seneviratne G . (2015). Developed fungal-bacterial biofilms having nitrogen fixers: universal biofertilizers for legumes and non-legumes de Bruijn FJ (ed). Biol Nitrogen Fixation. Wiley-Blackwell Press: Hoboken, NJ, USA, pp 1041–1046.
Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK et al. (2000). Quantification of biofilm structures by the novel computer program comstat. Microbiology 146: 2395–2407.
Hogan DA, Kolter R . (2002). Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296: 2229–2232.
Huergo LF, Dixon R . (2015). The emergence of 2-oxoglutarate as a master regulator metabolite. Microbiol Mol Biol Rev 79: 419–435.
Huerta JM, Aguilar I, Lopez-Pliego L, Fuentes-Ramirez LE, Castaneda M . (2016). The role of the ncRNA RgsA in the oxidative stress response and biofilm formation in Azotobacter vinelandii. Curr Microbiol 72: 671–679.
Inomura K, Bragg J, Follows MJ . (2017). A quantitative analysis of the direct and indirect costs of nitrogen fixation: a model based on Azotobacter vinelandii. ISME J 11: 166–175.
Lalucat J, Bennasar A, Bosch R, Garcia-Valdes E, Palleroni NJ . (2006). Biology of Pseudomonas stutzeri. Microbiol Mol Biol Rev 70: 510–547.
Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ . (2006). Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol 188: 8213–8221.
Matthew SB, Irina S, Evgueny V, Haiping L, April BS, Stephen HR et al. (2009). Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol 73: 622–638.
Meneses CH, Rouws LF, Simões-Araújo JL, Vidal MS, Baldani JI . (2011). Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus. Mol Plant-Microbe Interact 24: 1448–1458.
Okon Y, Albrecht SL, Burris RH . (1976). Factors affecting growth and nitrogen fixation of Spirillum lipoferum. J Bacteriol 127: 1248–1254.
Okon Y, Houchins JP, Albrecht SL, Burris RH . (1977). Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol 98: 87–93.
Pereg-Gerk L, Paquelin A, Gounon P, Kennedy IR, Elmerich C . (1998). A transcriptional regulator of the LuxR-UhpA family, FlcA, controls flocculation and wheat root surface colonization by Azospirillum brasilense Sp7. Mol Plant-Microbe Interact 11: 177–187.
Pope LM, Wyss O . (1970). Outer layers of the Azotobacter vinelandii cyst. J Bacteriol 102: 234–239.
Ramey BE, Koutsoudis M, von Bodman SB, Fuqua C . (2004). Biofilm formation in plant–microbe associations. Curr Opin Microbiol 7: 602–609.
Rediers H, Vanderleyden J, De Mot R . (2004). Azotobacter vinelandii: a Pseudomonas in disguise? Microbiology 150: 1117–1119.
Revers LF, Passaglia LM, Marchal K, Frazzon J, Blaha CG, Vanderleyden J et al. (2000). Characterization of an Azospirillum brasilense Tn5 mutant with enhanced N2 fixation: the effect of ORF280 on nifH expression. FEMS Microbiol Lett 183: 23–29.
Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS et al. (2007). The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci USA 104: 8113–8118.
Rinaudi LV, Giordano W . (2010). An integrated view of biofilm formation in rhizobia. FEMS Microbiol Lett 304: 1–11.
Rosche B, Li XZ, Hauer B, Schmid A, Buehler K . (2009). Microbial biofilms: a concept for industrial catalysis? Trends Biotechnol 27: 636–643.
Sadasivan L, Neyra CA . (1985). Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J Bacteriol 163: 716–723.
Sadasivan L, Neyra CA . (1987). Cyst production and brown pigment formation in aging cultures of Azospirillum brasilense ATCC 29145. J Bacteriol 169: 1670–1677.
Sadoff HL . (1975). Encystment and germination in Azotobacter vinelandii. Bacteriological Rev 39: 516.
Vermeiren H, Willems A, Schoofs G, de Mot R, Keijers V, Hai W et al. (1999). The rice inoculant strain Alcaligenes faecalis A15 is a nitrogen-fixing Pseudomonas stutzeri. Sys Appl Microbiol 22: 215–224.
Vlamakis H, Chai Y, Beauregard P, Losick R, Kolter R . (2013). Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol 11: 157–168.
Wang D, Zhang Y, Welch E, Li J, Roberts GP . (2010). Elimination of Rubisco alters the regulation of nitrogenase activity and increases hydrogen production in Rhodospirillum rubrum. Int J Hydrogen Energy 35: 7377–7385.
Wiig JA, Hu YL, Lee CC, Ribbe MW . (2012). Radical SAM-dependent carbon insertion into the nitrogenase M-cluster. Science 337: 1672–1675.
Yan Y, Yang J, Dou Y, Chen M, Ping S, Peng J et al. (2008). Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc Natl Acad Sci USA 105: 7564–7569.
Yan Y, Lu W, Chen M, Wang J, Zhang W, Zhang Y et al. (2013). Genome transcriptome analysis and functional characterization of a nitrogen-fixation island in root-associated Pseudomonas stutzeri. Mol Micro Ecol Rhizosp 1 and 2: 851–863.
Yang J, Xie X, Wang X, Dixon R, Wang YP . (2014). Reconstruction and minimal gene requirements for the alternative iron-only nitrogenase in Escherichia coli. Proc Natl Acad Sci USA 111: E3718–E3725.
You CB, Song HX, Wang JP, Lin M, Hai WL . (1991). Association of Alcaligenes faecalis with wetland rice. Plant Soil 137: 81–85.
Young JM, Leschine SB, Reguera G . (2012). Reversible control of biofilm formation by Cellulomonas spp. in response to nitrogen availability. Enviro Microbiol 14: 594–604.
Zadik Y, Yitschaky O, Neuman T, Nitzan DW . (2011). On the self-resolution nature of the buccal bifurcation cyst. J Oral Maxillofac Surg 69: e282–e284.
Acknowledgements
We thank Dr Min Lin from the Biotechnology Research Institute, Chinese Academy of Agricultural Sciences, for providing P. stutzeri strain. Mr Chunli Li and Ms Jingnan Liang from the Institute of Microbiology, Chinese Academy of Sciences for technical support on SEM and TEM, respectively. This work was supported by the National Basic Research Program of China (973 Program 2014CB846002 to LM and 2015CB150600), and the National Natural Science Foundation of China (31570126 to LM and 31200044 to DW). This work was conducted with the support of Institut Pasteur, Paris, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Wang, D., Xu, A., Elmerich, C. et al. Biofilm formation enables free-living nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions. ISME J 11, 1602–1613 (2017). https://doi.org/10.1038/ismej.2017.30
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2017.30
This article is cited by
-
Microbial aggregates and functional materials for mitigating soil nitrogen loss: a review
Reviews in Environmental Science and Bio/Technology (2026)
-
Enhancing Biological Nitrogen Fixation in Rice (Oryza sativa L.) Cultivation Through Diazotrophs-Enriched Periphyton Biofilm
Journal of Plant Growth Regulation (2026)
-
Biofilms: from the cradle of life to life support
npj Biofilms and Microbiomes (2026)
-
Non-cyanobacterial diazotrophs support the survival of marine microalgae in nitrogen-depleted environment
Genome Biology (2025)
-
Taxonomic and functional differentiation of soil and thallus microbiomes in Riccia sorocarpa
Biologia (2025)