Abstract
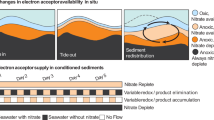
Nitrogen (N) input to the coastal oceans has increased considerably because of anthropogenic activities, however, concurrent increases have not occurred in open oceans. It has been suggested that benthic denitrification in sandy coastal sediments is a sink for this N. Sandy sediments are dynamic permeable environments, where electron acceptor and donor concentrations fluctuate over short temporal and spatial scales. The response of denitrifiers to these fluctuations are largely unknown, although previous observations suggest they may denitrify under aerobic conditions. We examined the response of benthic denitrification to fluctuating oxygen concentrations, finding that denitrification not only occurred at high O2 concentrations but was stimulated by frequent switches between oxic and anoxic conditions. Throughout a tidal cycle, in situtranscription of genes for aerobic respiration and denitrification were positively correlated within diverse bacterial classes, regardless of O2 concentrations, indicating that denitrification gene transcription is not strongly regulated by O2 in sandy sediments. This allows microbes to respond rapidly to changing environmental conditions, but also means that denitrification is utilized as an auxiliary respiration under aerobic conditions when imbalances occur in electron donor and acceptor supply. Aerobic denitrification therefore contributes significantly to N-loss in permeable sediments making the process an important sink for anthropogenic N-inputs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmerkamp S, Winter C, Janssen F, Kuypers MMM, Holtappels M . (2015). The impact of bedform migration on benthic oxygen fluxes. J Geophys Res Biogeosci 120: 2229–2242.
Arai H . (2011). Regulation and function of versatile aerobic and anaerobic respiratory metabolism inPseudomonas aeruginosa. Front Microbiol 2: 103.
Bakken LR, Bergaust L, Liu B, Frostegård Å . (2012). Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils. Philos Trans R Soc B Biol Sci 367: 1226–1234.
Baumann B, Snozzi M, Zehnder AJB, vanderMeer JR . (1996). Dynamics of denitrification activity of Paracoccus denitrificans in continuous culture during aerobic-anaerobic changes. J Bacteriol 178: 4367–4374.
Baumann B, van der Meer JR, Snozzi M, Zehnder AJ . (1997). Inhibition of denitrification activity but not of mRNA induction in Paracoccus denitrificans by nitrite at a suboptimal pH. Antonie van Leeuwenhoek 72: 183–189.
Bell LC, Richardson DJ, Ferguson SJ . (1990). Periplasmic and membrane-bound respiratory nitrate reductases in Thiosphaera pantotropha. FEBS Lett 265: 85–87.
Bergaust L, Shapleigh J, Frostegard A, Bakken L . (2008). Transcription and activities of NOx reductases in Agrobacterium tumefaciens: the influence of nitrate, nitrite and oxygen availability. Environ Microbiol 10: 3070–3081.
Bergaust L, Mao Y, Bakken LR, Frostegård Å . (2010). Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrous oxide reductase in Paracoccus denitrificans. Appl Environ Microbiol 76: 6387–6396.
Bergaust L, van Spanning RJM, Frostegård Å, Bakken LR . (2012). Expression of nitrous oxide reductase in Paracoccus denitrificans is regulated by oxygen and nitric oxide through FnrP and NNR. Microbiology 158: 826–834.
Billerbeck M, Werner U, Polerecky L, Walpersdorf E, de Beer D, Huettel M . (2006). Surficial and deep pore water circulation governs spatial and temporal scales of nutrient recycling in intertidal sand flat sediment. Mar Ecol-Prog Ser 326: 61–76.
Böer SI, Arnosti C, van Beusekom JEE, Boetius A . (2009). Temporal variations in microbial activities and carbon turnover in subtidal sandy sediments. Biogeosciences 6: 1149–1165.
Bolger AM, Lohse M, Usadel B . (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics: btu170 30: 2114–2120.
Cardenas MB, Cook PL, Jiang H, Traykovski P . (2008). Constraining denitrification in permeable wave-influenced marine sediment using linked hydrodynamic and biogeochemical modeling. Earth Planet Sci Lett 275: 127–137.
Chen JW, Strous M . (2013). Denitrification and aerobic respiration, hybrid electron transport chains and co-evolution. Biochim Biophys Acta-Bioenerg 1827: 136–144.
Dalsgaard T, Stewart FJ, Thamdrup B, De Brabandere L, Revsbech NP, Ulloa O et al. (2014). Oxygen at nanomolar levels reversibly suppresses process rates and gene expression in anammox and denitrification in the oxygen minimum zone off Northern Chile. mBio 5: e01966.
de Beer D, Wenzhofer F, Ferdelman TG, Boehme SE, Huettel M, van Beusekom JEE et al. (2005). Transport and mineralization rates in North Sea sandy intertidal sediments, Sylt-Romo Basin, Wadden Sea. Limnol Oceanogr 50: 113–127.
Devol AH . (2015). Denitrification, anammox, and N(2) production in marine sediments. Ann Rev Mar Sci 7: 403–423.
Dong LF, Smith CJ, Papaspyrou S, Stott A, Osborn AM, Nedwell DB . (2009). Changes in benthic denitrification, nitrate ammonification, and anammox process rates and nitrate and nitrite reductase gene abundances along an estuarine nutrient gradient (the Colne Estuary, United Kingdom). Appl Environ Microbiol 75: 3171–3179.
Eggleston EM, Lee DY, Owens MS, Cornwell JC, Crump BC, Hewson I . (2015). Key respiratory genes elucidate bacterial community respiration in a seasonally anoxic estuary. Environ Microbiol 17: 2306–2318.
Ehrenhauss S, Witte U, Buhring SI, Huettel M . (2004a). Effect of advective pore water transport on distribution and degradation of diatoms in permeable North Sea sediments. Mar Ecol Prog Ser 271: 99–111.
Ehrenhauss S, Witte U, Janssen F, Huettel M . (2004b). Decomposition of diatoms and nutrient dynamics in permeable North Sea sediments. Cont Shelf Res 24: 721–737.
Ellington MJ, Bhakoo KK, Sawers G, Richardson DJ, Ferguson SJ . (2002). Hierarchy of carbon source selection in Paracoccus pantotrophus: strict correlation between reduction state of the carbon substrate and aerobic expression of the nap operon. J Bacteriol 184: 4767–4774.
Emery KO . (1968). Relict sediments on continental shelves of world. AAPG Bull 52: 445–464.
Frette L, Gejlsbjerg B, Westermann P . (1997). Aerobic denitrifiers isolated from an alternating activated sludge system. FEMS Microbiol Ecol 24: 363–370.
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP et al. (2004). Nitrogen cycles: past, present, and future. Biogeochemistry 70: 153–226.
Gao H, Schreiber F, Collins G, Jensen MM, Kostka JE, Lavik G et al. (2010). Aerobic denitrification in permeable Wadden Sea sediments. ISME J 4: 417–426.
Gao H, Matyka M, Liu B, Khalili A, Kostka JE, Collins G et al. (2012). Intensive and extensive nitrogen loss from intertidal permeable sediments of the Wadden Sea. Limnol Oceanogr 57: 185–198.
Gihring TM, Canion A, Riggs A, Huettel M, Kostka JE . (2010). Denitrification in shallow, sublittoral Gulf of Mexico permeable sediments. Limnol Oceanogr 55: 43–54.
Gruber N, Galloway JN . (2008). An Earth-system perspective of the global nitrogen cycle. Nature 451: 293–296.
Hall SJ . (2002). The continental shelf benthic ecosystem: current status, agents for change and future prospects. Environ Conserv 29: 350–374.
Hewson I, Eggleston EM, Doherty M, Lee DY, Owens M, Shapleigh JP et al. (2014). Metatranscriptomic analyses of plankton communities inhabiting surface and subpycnocline waters of the Chesapeake Bay during oxic-anoxic-oxic transitions. Appl Environ Microbiol 80: 328–338.
Hojberg O, Binnerup SJ, Sorensen J . (1997). Growth of silicone-immobilized bacteria on polycarbonate membrane filters, a technique to study microcolony formation under anaerobic conditions. Appl Environ Microbiol 63: 2920–2924.
Huettel M, Rusch A . (2000). Transport and degradation of phytoplankton in permeable sediment. Limnol Oceanogr 45: 534–549.
Huettel M, Roy H, Precht E, Ehrenhauss S . (2003). Hydrodynamical impact on biogeochemical processes in aquatic sediments. Hydrobiologia 494: 231–236.
Huettel M, Berg P, Kostka JE . (2014). Benthic exchange and biogeochemical cycling in permeable sediments. Annu Rev Mar Sci 6: 23–51.
Huson DH, Mitra S, Ruscheweyh H-J, Weber N, Schuster SC . (2011). Integrative analysis of environmental sequences using MEGAN4. Genome Res 21: 1552–1560.
Hyatt D, Chen G-L, LoCascio PF, Land ML, Larimer FW, Hauser LJ . (2010). Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinform 11: 1–11.
Jansen S, Walpersdorf E, Werner U, Billerbeck M, Bottcher ME, de Beer D . (2009). Functioning of intertidal flats inferred from temporal and spatial dynamics of O2, H2S and pH in their surface sediment. Ocean Dyn 59: 317–332.
Ji B, Yang K, Zhu L, Jiang Y, Wang H, Zhou J et al. (2015). Aerobic denitrification: a review of important advances of the last 30 years. Biotechnol Bioprocess Eng 20: 643–651.
Ka JO, Urbance J, Ye RW, Ahn TY, Tiedje JM . (1997). Diversity of oxygen and N-oxide regulation of nitrite reductases in denitrifying bacteria. FEMS Microbiol Lett 156: 55–60.
Kalvelage T, Lavik G, Jensen MM, Revsbech NP, Löscher C, Schunck H et al. (2015). Aerobic microbial respiration in oceanic oxygen minimum zones. PLoS One 10: e0133526.
Kim M, Jeong S-Y, Yoon SJ, Cho SJ, Kim YH, Kim MJ et al. (2008). Aerobic denitrification of Pseudomonas putida AD-21 at different C/N ratios. J Biosci Bioeng 106: 498–502.
Kopylova E, Noé L, Touzet H . (2012). SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28: 3211–3217.
Li W, Godzik A . (2006). Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–1659.
Marchant HK, Lavik G, Holtappels M, Kuypers MMM . (2014). The fate of nitrate in intertidal permeable sediments. PLoS One 9: e104517.
Marchant HK, Holtappels M, Lavik G, Ahmerkamp S, Winter C, Kuypers MMM . (2016). Coupled nitrification–denitrification leads to extensive N loss in subtidal permeable sediments. Limnol Oceanogr 61: 1033–1048.
Morris RL, Schmidt TM . (2013). Shallow breathing: bacterial life at low O2. Nat Rev Micro 11: 205–212.
Nielsen LP . (1992). Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol Ecol 86: 357–362.
Otten MF, Stork DM, Reijnders WN, Westerhoff HV, Van Spanning RJ . (2001). Regulation of expression of terminal oxidases in Paracoccus denitrificans. Eur J Biochem 268: 2486–2497.
Patureau D, Davison J, Bernet N, Moletta R . (1994). Denitrification under various aeration conditions in Comamonas sp. strain SGLY2. FEMS Microbiol Ecol 14: 71–78.
Patureau D, Zumstein E, Delgenes JP, Moletta R . (2000). Aerobic denitrifiers isolated from diverse natural and managed ecosystems. Microbial Ecol 39: 145–152.
Pilditch CA, Miller DC . (2006). Phytoplankton deposition to permeable sediments under oscillatory flow: effects of ripple geometry and resuspension. Cont Shelf Res 26: 1806–1825.
Polerecky L, Franke U, Werner U, Grunwald B, de Beer D . (2005). High spatial resolution measurement of oxygen consumption rates in permeable sediments. Limnol Oceanogr Methods 3: 75–85.
Poole RK, Cook GM . (2000). Redundancy of aerobic respiratory chains in bacteria? Routes, reasons and regulation. Adv Microbial Physiol 43: 165–224.
Qu Z, Bakken LR, Molstad L, Frostegård Å, Bergaust LL . (2016). Transcriptional and metabolic regulation of denitrification in Paracoccus denitrificans allows low but significant activity of nitrous oxide reductase under oxic conditions. Environ Microbiol 18: 2951–2963.
Rao AMF, McCarthy MJ, Gardner WS, Jahnke RA . (2008). Respiration and denitrification in permeable continental shelf deposits on the South Atlantic Bight: N2: Ar and isotope pairing measurements in sediment column experiments. Cont Shelf Res 28: 602–613.
Reuter R, Badewien TH, Bartholomä A, Braun A, Lübben A, Rullkötter J . (2009). A hydrographic time series station in the Wadden Sea (southern North Sea). Ocean Dyn 59: 195–211.
Richardson D . (2008) Structural and functional flexibility of bacterial respiromes. In: El-Sharoud W (ed), Bacterial Physiology. Springer: Berlin Heidelberg, Germany, pp 97–128.
Richardson DJ, Ferguson SJ . (1992). The influence of carbon substrate on the activity of the periplasmic nitrate reductase in aerobically grown Thiosphaera pantotropha. Arch Microbiol 157: 535–537.
Robertson LA, Kuenen JG . (1984). Aerobic denitrification - old wine in new bottles. Antonie Van Leeuwenhoek J Microbiol 50: 525–544.
Robertson LA, Kuenen JG . (1990). Combined heterotrophic nitrification and aerobic denitrification in Thiosphaera pantotropha and other bacteria. Antonie van Leeuwenhoek 57: 139–152.
Robertson LA, Dalsgaard T, Revsbech NP, Kuenen JG . (1995). Confirmation of aerobic denitrification in batch cultures, using gas-chromatography and N15 mass spectrometry. FEMS Microbiol Ecol 18: 113–119.
Rusch A, Forster S, Huettel M . (2001). Bacteria, diatoms and detritus in an intertidal sandflat subject to advective transport across the water-sediment interface. Biogeochemistry 55: 1–27.
Schunck H, Lavik G, Desai DK, Großkopf T, Kalvelage T, Löscher CR et al. (2013). Giant hydrogen sulfide plume in the oxygen minimum zone off Peru supports chemolithoautotrophy. PLoS One 8: e68661.
Sears HJ, Spiro S, Richardson DJ . (1997). Effect of carbon substrate and aeration on nitrate reduction and expression of the periplasmic and membrane-bound nitrate reductases in carbon-limited continuous cultures of Paracoccus denitrificans Pd1222. Microbiology 143: 3767–3774.
Smith CJ, Nedwell DB, Dong LF, Osborn AM . (2007). Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl Environ Microbiol 73: 3612–3622.
Sokoll S, Lavik G, Sommer S, Goldhammer T, Kuypers MMM, Holtappels M . (2016). Extensive nitrogen loss from permeable sediments off North-West Africa. J Geophys Res Biogeosci 121: 1144–1157.
Sousa FL, Alves RJ, Pereira-Leal JB, Teixeira M, Pereira MM . (2011). A bioinformatics classifier and database for heme-copper oxygen reductases. PLoS One 6: e19117.
Stief P, Kamp A, de Beer D . (2013). Role of diatoms in the spatial-temporal distribution of intracellular nitrate in intertidal sediment. PLoS One 8: e73257.
Tseng C-P, Albrecht J, Gunsalus RP . (1996). Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol 178: 1094–1098.
Vetriani C, Voordeckers JW, Crespo-Medina M, O'Brien CE, Giovannelli D, Lutz RA . (2014). Deep-sea hydrothermal vent Epsilonproteobacteria encode a conserved and widespread nitrate reduction pathway (Nap). ISME J 8: 1510–1521.
Zennaro E, Ciabatti I, Cutruzzola F, D'Alessandro R, Silvestrini MC . (1993). The nitrite reductase gene of Pseudomonas aeruginosa: effect of growth conditions on the expression and construction of a mutant by gene disruption. FEMS Microbiol Lett 109: 243–250.
Zhou J, Bruns MA, Tiedje JM . (1996). DNA recovery from soils of diverse composition. Appl Environ Microbiol 62: 316–322.
Zumft WG . (1997). Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61: 533–616.
Acknowledgements
We thank the Marine Sensor Systems group at the ICBM, Oldenburg for providing data from the Time Series Station at Spiekeroog. G Klockgether for technical assistance, D de beer for assistance with the microsensor profiles and F Widdel for discussions concerning the data. This work was financially supported by the Max Planck Society and the DFG-Research Center/Cluster of Excellence 'The Ocean in the Earth System' at the University of Bremen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Marchant, H., Ahmerkamp, S., Lavik, G. et al. Denitrifying community in coastal sediments performs aerobic and anaerobic respiration simultaneously. ISME J 11, 1799–1812 (2017). https://doi.org/10.1038/ismej.2017.51
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2017.51
This article is cited by
-
Beyond dikarya: 28S metabarcoding uncovers cryptic fungal lineages across a tidal estuary
Environmental Microbiome (2025)
-
Long-term multi-meta-omics resolves the ecophysiological controls of seasonal N2O emissions during wastewater treatment
Nature Water (2025)
-
Microenvironments on individual sand grains enhance nitrogen loss in coastal sediments
Scientific Reports (2025)
-
Implications of climate change on biogeochemical cycles in the Arctic Ocean with special emphasis on the nitrogen cycle
Polar Biology (2025)
-
Growth of sulfate-reducing Desulfobacterota and Bacillota at periodic oxygen stress of 50% air-O2 saturation
Microbiome (2024)