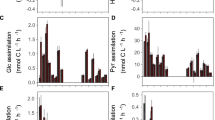
Abstract
Aerobic anoxygenic phototrophic (AAP) bacteria are microorganisms that can harvest light energy using bacteriochlorophyll a to supplement their predominantly organotrophic metabolism. Growth enhancement by light has repeatedly been demonstrated in laboratory experiments with AAP isolates. However, the ecological advantage of light utilization is unclear, as it has never been proven in the natural environment. Here, we conducted manipulation experiments in the NW Mediterranean and found that AAP bacteria display high growth rates which are controlled to a large extent by intense grazing pressure and phosphorous availability. Foremost, we found that, contrarily to the bulk bacterioplakton, AAP bacteria display higher growth rates when incubated under light-dark cycles than in complete darkness. These results represent the first direct evidence that natural populations of marine AAP bacteria can be stimulated by light.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Biebl H, Wagner-Döbler I . (2006). Growth and bacteriochlorophyll a formation in taxonomically diverse aerobic anoxygenic phototrophic bacteria in chemostat culture: influence of light regimen and starvation. Process Biochem 41: 2153–2159.
Cepáková Z, Hrouzek P, Žišková E, Nuyanzina-Boldareva E, Šorf M, Kozlíková-Zapomělová E et al. (2016). High turnover rates of aerobic anoxygenic phototrophs in European freshwater lakes. Environ Microbiol 18: 5063–5071.
Ferrera I, Borrego CM, Salazar G, Gasol JM . (2014). Marked seasonality of aerobic anoxygenic phototrophic bacteria in the coastal NW Mediterranean Sea as revealed by cell abundance, pigment concentration and pyrosequencing of pufM gene. Environ Microbiol 16: 2953–2965.
Ferrera I, Gasol JM, Sebastián M, Hojerová E, Kobížek M . (2011). Comparison of growth rates of aerobic anoxygenic phototrophic bacteria and other bacterioplankton groups in coastal mediterranean waters. Appl Environ Microbiol 77: 7451–7458.
Garcia-Chaves MC, Cottrell MT, Kirchman DL, Ruiz-González C, del Giorgio PA . (2016). Single-cell activity of freshwater aerobic anoxygenic phototrophic bacteria and their contribution to biomass production. ISME J 10: 1579–1588.
Hauruseu D, Koblížek M . (2012). Influence of light on carbon utilization in aerobic anoxygenic phototrophs. Appl Environ Microbiol 78: 7414–7419.
Hojerová E, Mašín M, Brunet C, Ferrera I, Gasol JM, Koblížek M . (2011). Distribution and growth of aerobic anoxygenic phototrophs in the Mediterranean Sea. Environ Microbiol 13: 2717–2725.
Kirchman DL, Hanson TE . (2013). Bioenergetics of photoheterotrophic bacteria in the oceans. Environ Microbiol Rep 5: 188–199.
Koblížek M . (2015). Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol Rev 39: 854–870.
Koblížek M, Mašín M, Ras J, Poulton AJ, Prášil O . (2007). Rapid growth rates of aerobic anoxygenic phototrophs in the ocean. Environ Microbiol 9: 2401–2406.
Krom MD, Kress N, Brenner S, Gordon LI . (1991). Phosphorus limitation of primary productivity in the Eastern Mediterranean Sea. Limnol Oceanogr 36: 424–432.
Lamy D, De Carvalho-Maalouf P, Cottrell MT, Lami R, Catala P, Oriol L et al. (2011). Seasonal dynamics of aerobic anoxygenic phototrophs in a Mediterranean coastal lagoon. Aquat Microb Ecol 62: 153–163.
Liu R, Zhang Y, Jiao N . (2010). Diel variations in frequency of dividing cells and abundance of aerobic anoxygenic phototrophic bacteria in a coral reef system of the South China Sea. Aquat Microb Ecol 58: 303–310.
Pinhassi J, Delong EF, Béjà O, González JM, Pedrós-alió C . (2016). Marine bacterial and archaeal ion-pumping rhodopsins: genetic diversity. Physiol Ecol 80: 929–954.
Ruiz-González C, Simó R, Sommaruga R, Gasol JM . (2013). Away from darkness: a review on the effects of solar radiation on heterotrophic bacterioplankton activity. Front Microbiol 4: 1–24.
Schwalbach MS, Brown M, Fuhrman JA . (2005). Impact of light on marine bacterioplankton community structure. Aquat Microb Ecol 39: 235–245.
Stegman MR, Cottrell MT, Kirchman DL . (2014). Leucine incorporation by aerobic anoxygenic phototrophic bacteria in the Delaware estuary. ISME J 8: 2339–2348.
Yurkov VV, van Gemerden H . (1993). Impact of light/dark regimen on growth rate, biomass formation and bacteriochlorophyll synthesis in Erythromicrobium hydrolyticum. Arch Microbiol 159: 84–89.
Acknowledgements
This work was supported by grant REMEI (CTM2015-70340-R) from the Spanish Ministry of Economy, Industry and Competitivity. MK was supported by the GA ČR project 13-11281S and the MŠMT project Algatech Plus.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Ferrera, I., Sánchez, O., Kolářová, E. et al. Light enhances the growth rates of natural populations of aerobic anoxygenic phototrophic bacteria. ISME J 11, 2391–2393 (2017). https://doi.org/10.1038/ismej.2017.79
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ismej.2017.79
This article is cited by
-
Mapping the metagenomic diversity of the multi-kingdom glacier-fed stream microbiome
Nature Microbiology (2025)
-
Ecology of aerobic anoxygenic phototrophs on a fine-scale taxonomic resolution in Adriatic Sea unravelled by unsupervised neural network
Environmental Microbiome (2024)
-
Phenology and ecological role of aerobic anoxygenic phototrophs in freshwaters
Microbiome (2024)
-
Radiation impacts gene redundancy and biofilm regulation of cryoconite microbiomes in Northern Hemisphere glaciers
Microbiome (2023)
-
Community structure of aerobic anoxygenic phototrophic bacteria in algae- and macrophyte-dominated areas in Taihu Lake, China
Journal of Oceanology and Limnology (2022)