Abstract
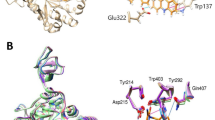
In situ click chemistry is a target-guided synthesis technique for discovering potent protein ligands by assembling azides and alkynes into triazoles inside the affinity site of a target protein. We report the rapid discovery of a new and potent inhibitor of bacterial chitinases by the use of in situ click chemistry. We observed a target-templated formation of a potent triazole inhibitor of the chitinase-catalyzed chitin hydrolysis, through in situ click chemistry between a biologically active azide-containing scaffold and structurally unrelated alkyne fragments. Chitinase inhibitors have chemotherapeutic potential as fungicides, pesticides and antiasthmatics. Argifin, which has been isolated and characterized as a cyclopentapeptide natural product by our research group, shows strong inhibitory activity against chitinases. As a result of our efforts at developing a chitinase inhibitor from an azide-bearing argifin fragment and the application of the chitinase template and a library of alkynes, we rapidly obtained a very potent and new 1,5-disubstituted triazole inhibitor against Serratia marcescens chitinase (SmChi) B. The new inhibitor expressed 300-fold increase in the inhibitory activity against SmChiB compared with that of argifin. To the best of our knowledge, our finding of an enzyme-made 1,5-disubstituted triazole, using in situ click chemistry is the second example reported in the literature.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Boot, R. G. et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J. Biol. Chem. 276, 6770–6778 (2001).
Shahabuddin, M., Toyoshima, T., Aikawa, M. & Kaslow, D. C. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc. Natl Acad. Sci. USA 90, 4266–4270 (1993).
Shibata, Y., Foster, L. A., Bradfield, J. F. & Myrvik, Q. N. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J. Immunol. 164, 1314–1321 (2000).
Andersen, O. A., Dixon, M. J., Eggleston, I. M. & van Aalten, D. M. F. Natural product family 18 chitinase inhibitors. Nat. Prod. Rep. 22, 563–579 (2005).
Zhu, Z. et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304, 1678–1682 (2004).
Ōmura, S. et al. Argifin, a new chitinase inhibitor, produced by Gliodadium sp. FTD-0668. I. Taxonomy, fermentation, and biological activities. J. Antibiot. 53, 603–608 (2000).
Arai, N., Shiomi, K., Iwai, Y. & Ōmura, S. Argifin, a new chitinase inhibitor, produced by Gliodadium sp. FTD-0668. II. Isolation, physico-chemical properties, and structure elucidation. J. Antibiot. 53, 609–614 (2000).
Shiomi, K. et al. Structure of argifin, a new chitinase inhibitor produced by Gliocladium sp. Tetrahedron Lett. 41, 2141–2143 (2000).
Suzuki, K. et al. Chitinases A, B, and C1 of Serratia marcescens 2170 produced by recombinant Escherichia coli: enzymatic properties and synergism on chitin degradation. Biosci. Biotechnol. Biochem. 66, 1075–1083 (2002).
Horn, S. J. et al. Comparative studies of chitinases A, B and C from Serratia marcescens. Biocatal. Biotransform. 24, 39–53 (2006).
Rao, F. V. et al. Specificity and affinity of natural product cyclopentapeptide inhibitors against A. fumigatus, human, and bacterial chitinases. Chem. Biol. 12, 65–76 (2005).
Houston, D. R. et al. High-resolution structures of a chitinase complexed with natural product cyclopentapeptide inhibitors: mimicry of carbohydrate substrate. Proc. Natl Acad. Sci. USA 99, 9127–9132 (2002).
Andersen, O. A. et al. Structure-based dissection of the natural product cyclopentapeptide chitinase inhibitor argifin. Chem. Biol. 15, 295–301 (2008).
Rideout, D. Self-assembling cytotoxins. Science 233, 561–563 (1986).
Rideout, D., Calogeropoulu, T., Jaworski, J. & McCarthy, M. Synergism through direct covalent bonding between agents: a strategy for rational design of chemotherapeutic combinations. Biopolymers 29, 247–262 (1990).
Inglese, J. & Benkovic, S. J. Multisubstrate adduct inhibitors of glycinamide ribonucleotide transformylase: synthetic and enzyme-assembled. Tetrahedron 47, 2351–2364 (1991).
Boger, D. L. et al. 10-formyl-5,8,10-trideazafolic acid (10-formyl-TDAF): a potent inhibitor of glycinamide ribonucleotide transformylase. Bioorg. Med. Chem. 5, 1817–1830 (1997).
Maly, D. J., Choong, I. C. & Ellman, J. A. Combinatorial target-guided ligand assembly: identification of potent subtype-selective c-Src inhibitors. Proc. Natl Acad. Sci. USA 97, 2419–2424 (2000).
Nicolaou, K. C. et al. Target-accelerated combinatorial synthesis and discovery of highly potent antibiotics effective against vancomycin-resistant bacteria. Angew. Chem. Int. Ed. 39, 3823–3828 (2000).
Greasley, S. E. et al. Unexpected formation of an epoxide-derived multisubstrate adduct inhibitor on the active site of GAR transformylase. Biochemistry 40, 13538–13547 (2001).
Nguyen, R. & Huc, I. Using an enzyme's active site to template inhibitors. Angew. Chem. Int. Ed. 40, 1774–1776 (2001).
Nicolaou, K. C. et al. Synthesis and biological evaluation of vancomycin dimers with potent activity against vancomycin-resistant bacteria: target-accelerated combinatorial synthesis. Chem. Eur. J. 7, 3824–3843 (2001).
Kehoe, J. W. et al. Tyrosylprotein sulfotransferase inhibitors generated by combinatorial target-guided ligand assembly. Bioorg. Med. Chem. Lett. 12, 329–332 (2002).
Poulin-Kerstien, A. T. & Dervan, P. B. DNA-templated dimerization of hairpin polyamides. J. Am. Chem. Soc. 125, 15811–15821 (2003).
Hu, X., Sun, J., Wang, H.-G. & Manetsch, R. Bcl-XL-templated assembly of its own protein-protein interaction modulator from fragments decorated with thio acids and sulfonyl azides. J. Am. Chem. Soc. 130, 13820–13821 (2008).
Huisgen, R. in 1,3-Dipolar Cycloaddition Chemistry, Vol. 1 (ed. Padwa, A.) 1–176 (Wiley, New York, 1984).
Sharpless, K. B. & Manetsch, R. In situ click chemistry: a powerful means for lead discovery. Expert Opin. Drug Discov. 1, 525–538 (2006).
Lewis, W. G. et al. Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew. Chem. Int. Ed. 41, 1053–1057 (2002).
Manetsch, R. et al. In situ click chemistry: enzyme inhibitors made to their own specifications. J. Am. Chem. Soc. 126, 12809–12818 (2004).
Bourne, Y. et al. Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation. Proc. Natl Acad. Sci. USA 101, 1449–1454 (2004).
Krasiéski, A. et al. In situ selection of lead compounds by click chemistry: target-guided optimization of acetylcholinesterase inhibitors. J. Am. Chem. Soc. 127, 6686–6692 (2005).
Mocharla, V. P. et al. In situ click chemistry: enzyme-generated inhibitors of carbonic anhydrase II. Angew. Chem. Int. Ed. 44, 116–120 (2005).
Whiting, M. et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. 45, 1435–1439 (2006).
Brurberg, M. B. et al. Chitinase B from Serratia marcescens BJL200 is exported to the periplasm without processing. Microbiology 141, 123–131 (1995).
Hodge, A., Gooday, G. W. & Alexander, I. J. Inhibition of chitinolytic activities from tree species and associated fungi. Phytochemistry 41, 77–84 (1996).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Chan, T. R., Hilgraf, R., Sharpless, K. B. & Fokin, V. V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 6, 2853–2885 (2004).
Zhang, L. et al. Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc. 127, 15998–15999 (2005).
Boren, B. C. et al. Ruthenium-catalyzed azide-alkyne cycloaddition: scope and mechanism. J. Am. Chem. Soc. 130, 8923–8930 (2008).
Arai, N. et al. Argadin, a new chitinase inhibitor, produced by Clonostachys sp. FO-7314. Chem. Pharm. Bull. 48, 1442–1446 (2000).
Acknowledgements
This work was supported by the Grant of the 21st Century COE Program, Ministry of Education Culture, Sports, Science and Technology. We also thank Ms A Nakagawa, Ms N Sato and Dr K Nagai for various instrumental analyses.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website (http://www.nature.com/ja)
Supplementary information
Rights and permissions
About this article
Cite this article
Hirose, T., Sunazuka, T., Sugawara, A. et al. Chitinase inhibitors: extraction of the active framework from natural argifin and use of in situ click chemistry. J Antibiot 62, 277–282 (2009). https://doi.org/10.1038/ja.2009.28
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2009.28
Keywords
This article is cited by
-
Marine chitinolytic enzymes, a biotechnological treasure hidden in the ocean?
Applied Microbiology and Biotechnology (2018)
-
In situ click chemistry generation of cyclooxygenase-2 inhibitors
Nature Communications (2017)
-
A new application of click chemistry in situ: development of fluorescent probe for specific G-quadruplex topology
Scientific Reports (2015)
-
Catalytic site inhibition of insulin-degrading enzyme by a small molecule induces glucose intolerance in mice
Nature Communications (2015)
-
Solid-phase total synthesis of the chitinase inhibitor Argadin using a supported acetal resin
The Journal of Antibiotics (2009)