Abstract
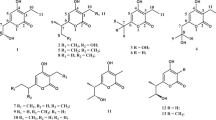
From the wild-type strain Steptomyces sp. AK 671, three nitrogen-containing octaketides were isolated, bhimamycins F, H and I, besides the known azaanthraquinone utahmycin A and polyketide shunt products SEK 4, SEK 4b, mutactin, dehydromutactin and EM18. The structures were characterized by MS and NMR experiments. The hitherto unknown absolute configuration of the two enantiomers of EM18 was determined by online-CD spectroscopy and quantum-chemical CD calculations. Bhimamycins H and I show weak antibacterial activities, whereas the enzyme phosphodiesterase 4 is strongly inhibited by bhimamycins H and I, which has never been reported for nitrogen-containing octaketides. In addition, bhimamycin H inhibits the enzyme glycogen synthase kinase-3β.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Helaly, E. H. et al. Warkmycin, a novel angucycline antibiotic produced by Streptomyces sp. Acta 2930. J. Antibiot. (e-pub ahead of print 17 July 2013; doi:10.1038/ja.2013.74).
Fiedler, H.-P. et al. Genoketides A1 and A2, new octaketides and biosynthetic intermediates of chrysophanol produced by Streptomyces sp. AK 671. J. Antibiot. 61, 464–473 (2008).
Bringmann, G. et al. Different polyketide folding modes converge to an identical molecular architecture. Nature Chem. Biol. 2, 429–433 (2006).
Bringmann, G., Gulder, T. A. M., Hamm, A., Goodfellow, M. & Fiedler, H.-P. Multiple convergence in polyketide biosynthesis: a third folding mode to anthraquinone chrysophanol. Chem. Commun. 2009, 6810–6812 (2009).
Kalaitzis, J. A. & Moore, B. S. Heterologous biosynthesis of truncated hexaketides derived from the actinorhodin polyketide synthase. Nat. Prod. Rep. 67, 1419–1422 (2004).
Krupa, J., Lessmann, H. & Lackner, H. Ein α-methylanthrachinon aus streptomyceten. Liebigs Ann. Chem. 1989, 699–701 (1989).
Bauer, J. D., King, R. W. & Brady, S. F. Utahmycins A and B, azaquinones produced by an environmental DNA clone. J. Nat. Prod. 73, 976–979 (2010).
Fotso, S. et al. Bhimamycin A∼E and bhimanone: isolation, structure elucidation, and biological activity of novel quinone antibiotics from a terrestrial streptomycete. J. Antibiot. 56, 931–941 (2003).
Alvarez, M. A., Fu, H., Khosla, C., Hopwood, D. A. & Bailey, J. E. Engineered biosynthesis of novel polyketides: properties of the whiE aromatase/cyclase. Nat. Biotechnol. 14, 335–338 (1996).
Xiang, L. K., John, A. & Moore, B. S. EncM, a versatile enterocin biosynthetic enzyme involved in Favorskii oxidative rearrangement, aldol condensation, and heterocycle-forming reactions. Proc. Natl Acad. Sci. USA 101, 15609–15614 (2004).
Zhang, H.-I. et al. Mutactin, a novel polyketide from Streptomyces coelicolor. Structure and biosynthetic relationship to actinorhodin. J. Org. Chem. 55, 1682–1684 (1990).
Seebach, D. & Prelog, V. The unambiguous specification of the steric course of asymmetric syntheses. Angew. Chem. Int. Ed. Engl. 21, 654–660 (1982).
Claridge, T. D. W. High-Resolution NMR Techniques in Organic Chemistry Vol. 20, 301–306 Tetrahedron Organic Chemistry Series: Oxford, UK, (2009).
Berova, N., Polavarapu, P. L., Nakanishi, K. & Woody, R. W. Comprehensive Chiroptical Spectroscopy Vol. 2, (John Wiley & Sons: Hoboken, USA, (2012).
Cohen, P. & Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell. Biol. 2, 769–776 (2001).
Bhat, R. V., Budd Haeberlein, S. L. & Avila, J. Glycogen synthase kinase 3: a drug target for CNS therapies. J. Neurochem. 89, 1313–1317 (2004).
Hopwood, D. A. Genetic contribution to understanding polyketide synthases. Chem. Rev. 97, 2465–2497 (1997).
Fu, H., Ebert-Koshla, S., Hopwood, D. A. & Khosla, C. Engineered biosynthesis of novel polyketides: dissection of the catalytic specificity of the act ketoreductase. J. Am. Chem. Soc. 116, 4166–4170 (1994).
Bartel, P. L. et al. Biosynthesis of anthraquinones by interspecies cloning of actinorhodin biosynthesis genes in streptomycetes: clarification of actinorhodin gene functions. J. Bacteriol. 172, 4816–4826 (1990).
Bringmann, G. & Pokorny, F. In: Cordell G. A., (Ed.) The Alkaloids Vol. 46, 127–271 Academic Press: New York, USA, (1995).
Bringmann, G., Wohlfarth, M., Rischer, H., Grüne, M. & Schlauer, J. A new biosynthetic pathway to alkaloids in plants: acetogenic isoquinolines. Angew. Chem. Int. Ed. Engl. 112, 1464–1466 (2000).
King, R. W., Bauer, J. D. & Brady, S. F. An environmental DNA-derived type II polyketide biosynthetic pathway encodes the biosynthesis of the novel pentacyclic polyketide, erdacin. Angew. Chem. Int. Ed. Engl. 48, 6257–6261 (2009).
Bringmann, G. Biomimetische Synthesen beider Molekülhälften der Ancistrocladus- und der Triphyophyllum-Alkaloide aus gemeinsamen Vorstufen. Liebigs Ann. Chem. 1985, 2126–2134 (1985).
Page, C. P. & Spina, D. Selective PDE inhibitors as novel treatments for respiratory diseases. Curr. Opin. Pharmacol. 12, 275–286 (2012).
Nachtigall, J. et al. Benzoxacystol, a benzoxazine-type enzyme inhibitor from the deep-sea strain Streptomyces sp. NTK 935. J. Antibiot. 64, 453–457 (2011).
Fiedler, H.-P. Screening for novel secondary metabolites by HPLC and UV-visible absorbance libraries. Nat. Prod. Lett. 2, 119–128 (1993).
Schulz, D. et al. Abenquines A-D: aminoquinone derivatives produced by Streptomyces sp. strain DB634. J. Antibiot. 64, 763–768 (2011).
Kim, B.-Y. et al. Elaiomycins B and C, novel alkylhydrazides produced by Streptomyces sp. BK 190. J. Antibiot. 64, 595–597 (2011).
Baki, A., Bielik, A., Molnar, L., Szendrei, G. & Keseru, G. M. A. A high throughput luminescent assay for glycogen synthase kinase-3beta inhibitors. Assay Drug Develop. Technol 5, 75–83 (2007).
Acknowledgements
We are grateful to Prof M Goodfellow, Newcastle University, for providing us the strain Streptomyces sp. AK 671. Financial support from the DFG (German Research Association, Sonderforschungsbereich 630 “Agents against Infectious Diseases”, Universiät Würzburg) and from the Ministry of Science, Economic affairs and Transport of Schleswig-Holstein, Kiel, of the project no. 12208009 “Aufbau einer Reinsubstanz-Bibliothek mariner Naturstoffe’ is gratefully acknowledged. We thank E Ruckdeschel and Dr M Grüne for the NMR experiments, F Dadrich and Dr M Büchner for the mass spectra, and F Witterauf for the measurement of the online-CD spectra (Universität Würzburg). JFI and JW are grateful to A Erhard (Kieler Wirkstoff-Zentrum) for performing the activity tests. PJ and HPF thank A Kulik (Universität Tübingen) for assistance in fermentations and HPLC-ESI-MS analysis.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Art. No. 67 in ‘Biosynthetic Capacities of Actinomycetes’. Art. No. 66: see Helaly et al.
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Jetter, P., Steinert, C., Knauer, M. et al. New bhimamycins from Streptomyces sp. AK 671. J Antibiot 66, 719–726 (2013). https://doi.org/10.1038/ja.2013.82
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2013.82
Keywords
This article is cited by
-
An aberrant metabolic flow toward early shunt products in the granaticin biosynthetic machinery of Streptomyces vietnamensis GIMV4.0001
The Journal of Antibiotics (2020)