Abstract
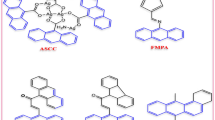
A high-throughput phenotypic screen for novel antibacterial agents led to the discovery of a novel pyrazolopyrimidinedione, PPD-1, with preferential activity against methicillin-resistant Staphylococcus aureus (MRSA). Resistance mapping revealed the likely target of inhibition to be lysyl tRNA synthetase (LysRS). Preliminary structure–activity relationship (SAR) studies led to an analog, PPD-2, which gained Gram-negative antibacterial activity at the expense of MRSA activity and resistance to this compound mapped to prolyl tRNA synthetase (ProRS). These targets of inhibition were confirmed in vitro, with PPD-1 showing IC50s of 21.7 and 35 μM in purified LysRS and ProRS enzyme assays, and PPD-2, 151 and 0.04 μM, respectively. The highly attractive chemical properties of these compounds combined with intriguing preliminary SAR suggest that further exploration of this compelling novel series is warranted.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Boucher, H. et al. Bad bugs no drugs, no ESKAPE. Clin. Infect. Dis. 48, 1–12 (2009).
Boucher, H. et al. 10 x '20 Progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin. Infect. Dis. 256, 1685–1694 (2013).
Centers for Disease Control and Prevention Executive Report: Antibiotic Resistance Threats in the United States (2013), http://www.cdc.gov/drugresistance/threat-report-2013/.
World Health Organization Antimicrobial resistance: global report on surveillance (2014), http://www.who.int/drugresistance/documents/surveillancereport/en/.
Silver, L. L . Challenges of antibacterial drug discovery. Clin. Microbiol. Rev. 24, 71–109 (2011).
Payne, D., Gwynn, M. N., Holmes, D. J . & Pompliano, D. L . Drugs for bad bugs, confronting the challenges of antimicrobial discovery. Nat. Rev. Drug Discov. 1, 29–40 (2007).
Miller, A. et al. Discovery and characterization of QPT-1, the progenitor of a new class of bacterial topoisomerase inhibitors. Antimicrob. Agents Chemother. 52, 2806–2812 (2008).
Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically 9th edn Vol. 32, CLSI: Wayne, PA, USA, (2012).
Murray, R. W., Melchior, E. P., Hagadorn, J. C . & Marotti, K. R . S. aureus cell extract transcription-translation assay: firefly luciferase reporter system for evaluating protein translation inhibitors. Antimicrob. Agents Chemother. 45, 1900–1904 (2001).
Hartman-Neumann, S., Dunham, S., Gosink, M., Kuhn, M . & Miller, A. Rapid elucidation of antibacterial targets and resistance determinants using an error-prone genomic library of N. gonorrhoeae, Poster F2-869, Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, USA (2010).
Shen, J., Wang, Y . & Schwarz, S . Presence and dissemination of the multiresistance gene cfr in Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 68, 1697–1706 (2013).
Dunkle, J. A., Xiong, L., Mankin, A. S . & Cate, J. H . Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl Acad. Sci. USA 107, 17152–17157 (2010).
Pollastri, M. P . Overview of the rule of five. Curr. Protoc. Pharmacol Chapter 9, Unit 9.12 (2010).
Lv, I. C . & Zhu, H. L . Aminoacyl-tRNA synthetase inhibitors as potent antibacterials. Curr. Med. Chem. 19, 3550–3563 (2012).
Pohlmann, J . & Brotz-Osterheltz, H . New aminoacyl tRNA synthetase inhibitors as antibacterial agents. Curr. Drug. Tar. Infect. Dis. 4, 261–272 (2004).
Gadakh, B . & VanAerschot, A . Aminoacyl-tRNA synthetase inhibitors as antimicrobial agents: a patent review from 2006 till present. Expert Opin. Ther. Pat. 22, 1453–1465 (2012).
Raczniak, G., Ibba, M . & Söll, D . Genomics-based identification of targets in pathogenic bacteria for potential therapeutic and diagnostic use. Toxicology 160, 181–189 (2001).
Zeng, Y. et al. Biosynthesis of albomycin δ2 provides a template for assembling siderophore and aminoacyl-tRNA synthetase inhibitor conjugates. ACS Chem. Biol. 7, 1565–1575 (2012).
Hurdle, J. G., O’Neill, A. J . & Chopra, I . Anti-staphylococcal activity of indolmycin, a potential topical agent for control of staphylococcal infections. J. Antimicrob. Chemother. 54, 549–552 (2004).
Severinov, K . & Nair, S. K . Microcin C: biosynthesis and mechanisms of bacterial resistance. Future Microbiol. 7, 281–289 (2012).
Ward, A . & Campoli-Richards, D. M . Mupirocin: a review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 32, 425–444 (1986).
Sutherland, R. et al. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob. Agents Chemother. 27, 495–498 (1985).
Sutcliffe, J. A . Antibiotics in development targeting protein synthesis. Ann. NY Acad. Sci. 1, 122–152 (2011).
Tenaro, D. et al. Intrapulmonary pharmacokinetics of GSK2251052 in healthy volunteers. Antimicrob. Agents Chemother. 57, 3334–3337 (2013).
Pucci, M. J . & Bush, K . Investigational antimicrobial agents of 2013. Clin. Microbiol. Rev. 26, 792–821 (2013).
Acknowledgements
We wish to acknowledge the former leaders of the Pfizer Antibacterial Research Unit for their unfailing dedication and support during very challenging times. We would also like to acknowledge our former colleague, Zuoyu Xu, for development of the staging assay during his time at Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All co-authors are current or former employees of Pfizer and thereby may own shares in the company.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Montgomery, J., Smith, J., Tomaras, A. et al. Discovery and characterization of a novel class of pyrazolopyrimidinedione tRNA synthesis inhibitors. J Antibiot 68, 361–367 (2015). https://doi.org/10.1038/ja.2014.163
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2014.163
This article is cited by
-
Are the physicochemical properties of antibacterial compounds really different from other drugs?
Journal of Cheminformatics (2016)