Abstract
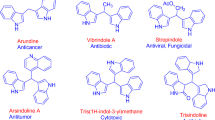
Chromatographic separation of a crude extract obtained from a fermentation broth of a chemically unknown bacterium Shewanella piezotolerans WP3 collected in deep-sea yielded three new indole alkaloids namely shewanellines A (1a), B (1b) and C (2), together with 12 known indole alkaloids. The structures were unambiguously elucidated on the basis of 1D and 2D NMR (1H, 13C, COSY, HMBC, HSQC and NOESY) in association with MS and CD data. Compounds 1–4, 7, 9 and 11–14 were selected for the evaluation of their cytotoxic activities against human tumor cell lines HL-60 and BEL-7402, whereas compounds 2, 4 and 9 exhibited significant inhibition toward HL-60.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Thornburg, C. C., Zabriskie, T. M. & McPhail, K. L. Deep-sea hydrothermal vents: potential hot spots for natural products discovery? J. Nat. Prod. 73, 489–499 (2010).
Skropeta, D. Deep-sea natural products. Nat. Prod. Rep. 25, 1131–1166 (2008).
Xiao, X., Wang, P., Zeng, X., Bartlett, D. H. & Wang, F. Shewanella psychrophila sp. nov. and Shewanella piezotolerans sp. nov., isolated from west Pacific deep-sea sediment. Int. J. Syst. Evol. Microbiol. 57, 60–65 (2007).
Wang, F., Wang, P., Chen, M. & Xiao, X. Isolation of extremophiles with the detection and retrieval of Shewanella strains in deep-sea sediments from the west Pacific. Extremophiles 8, 165–168 (2004).
Wang, F. et al. Environmental adaptation: genomic analysis of the piezotolerant and psychrotolerant deep-sea iron reducing bacterium Shewanella piezotolerans WP3. PLoS One 3, e1937 (2008).
Deng, J. et al. Facile creation of 3-indolyl-3-hydroxy-2-oxindoles by an organocatalytic enantioselective Friedel–Crafts reaction of indoles with isatins. Adv. Synth. Catal. 352, 833–838 (2010).
Chauhan, P. & Chimni, S. S. Asymmetric addition of indoles to isatins catalysed by bifunctional modified cinchona alkaloid catalysts. Chem. Eur. J. 16, 7709–7713 (2010).
Hanhan, N. V., Sahin, A. H., Chang, T. W., Fettinger, J. C. & Franz, A. K. Catalytic asymmetric synthesis of substituted 3-hydroxy-2-oxindoles. Angew. Chem. Int. Ed. 49, 744–747 (2010).
Ribe, S., Kondru, R. S., Beratan, D. N. & Wipf, P. Optical rotation computation, total synthesis, and stereochemistry assignment of the marine natural product pitiamide A. J. Am. Chem. Soc. 122, 4608–4617 (2000).
Smith, K., El-Hiti, G. A. & Hawes, A. C. Carbonylation of doubly lithiated N’-aryl-N,N-dimethylureas: a novel approach to isatins via intramolecular trapping of acyllithiums. Synthesis (Mass). 13, 2047–2052 (2003).
Kobayashi, M. et al. Marine natural products. XXXIV. trisindoline, a new antibiotic indole trimer, produced by a bacterium of Vibrio sp. separated from the marine sponge Hyrtios altum. Chem. Pharm. Bull. 42, 2449–2451 (1994).
Mizuno, T. & Ishino, Y. Highly efficient synthesis of 1H-quinazoline-2,4-diones using carbon dioxide in the presence of catalytic amount of DBU. Tetrahedron 58, 3155–3158 (2002).
Mohammadi, A. A., Dabiri, M. & Qaraat, H. A regioselective three-component reaction for synthesis of novel 1′H-spiro[isoindoline-1,2′-quinazoline]-3,4′(3′H) -dione derivatives. Tetrahedron 65, 3804–3808 (2009).
Hoessel, R. et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1, 60–67 (1999).
Kawasaki, T. et al. Synthesis of 3-hydroxyindolin-2-one alkaloids, (+)-donaxaridine and (+)-convolutamydines A and E, through enolization–Claisen rearrangement of 2-allyloxyindolin-3-ones. Tetrahedron 60, 3493–3503 (2004).
Tacconi, G. et al. 3-alkylidene-I,3-dihydroindol-2-ones: influence of configuration on the transmission of the inductive effect to the carbonyl group. J. Chem. Soc. Perkin Trans. 2, 150–154 (1976).
Huang, Z., Lui, D., Han, X., Zhang, Z. & Wang, J. Small molecule inhibitors of Bcl-2 proteins. WO Patent 2000004901 (2000).
Lu, L. et al. Studies on the constituents of Cimicifuga foetida collected in Guizhou province and their cytotoxic activities. Chem. Pharm. Bull. 60, 571–577 (2012).
Acknowledgements
This work was supported by grants from the NSFC (No.30930109), COMRA (DY125-15-T-01) and SOA (2010319123366025-4), and MOST 863 Project (2011AA090701, 2010DFA31610).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Y., Tang, X., Shao, Z. et al. Indole-based alkaloids from deep-sea bacterium Shewanella piezotolerans with antitumor activities. J Antibiot 67, 395–399 (2014). https://doi.org/10.1038/ja.2014.3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2014.3
Keywords
This article is cited by
-
Facile synthesis of unsymmetrical N-aryl-2,2-di(1H-indol-3-yl) acetamide derivatives
Chemical Research in Chinese Universities (2016)