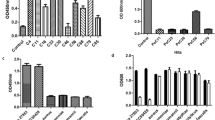
Abstract
The 14-membered macrolide erythromycin A expresses three distinct biological properties, including antibacterial activity, gastrointestinal motor-stimulating activity and anti-inflammatory and/or immunomodulatory effects. Although low-dose, long-term therapy using 14- and 15-membered macrolides displaying anti-inflammatory and/or immunomodulatory activity effectively treats diffuse panbronchiolitis and chronic sinusitis, bacterial resistance may emerge. To address this issue, we developed the 12-membered non-antibiotic macrolide (8R,9S)-8,9-dihydro-6,9-epoxy-8,9-anhydropseudoerythromycin A (EM900) that promotes monocyte to macrophage differentiation, a marker for anti-inflammatory and/or immunomodulatory effects, without possessing antibacterial activity. In this article, we report that the new macrolide derivative (8R,9S) -de(3’-N-methyl)-3’-N-(p-chlorobenzyl)-de(3-O-cladinosyl)-3-dehydro-8,9-dihydro-6,9-epoxy-8,9-anhydropseudoerythromycin A 12,13-carbonate (EM939) exhibited stronger promotive activity for monocyte to macrophage differentiation than that of the parent compound EM900 in addition to reduced cytotoxicity toward THP-1 cells and antibacterial inactivity. In a cigarette-smoking model used to simulate chronic obstructive pulmonary disease (COPD), the EM900 derivatives significantly attenuated lung and alveolar inflations, functionally and histologically, via oral administration. Because of these marked therapeutic effects, non-antibiotic EM900 derivatives may become central to the treatment of chronic inflammatory diseases such as COPD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sunazuka, T., Omura, S., Iwasaki, S. & Ōmura, S. in Macrolide Antibiotics. Chemistry, Biology, and Practice. 2nd edn, (ed. Ōmura S) Ch. 3, 99–180 (Academic press, California, 2002.
Itoh, Z., Nakaya, M., Suzuki, H., Arai, H. & Wakabayashi, K. Erythromycin mimics exogenous motilin in gastrointestinal contractile activity in the dog. Am. J. Physiol. 247, G688–G694 (1984).
Inatomi, N., Sato, F., Itoh, Z. & Ōmura, S. in Macrolide Antibiotics. Chemistry, Biology, and Practice 2nd edn. 2nd edn, (ed. Ōmura S) Ch. 11, 501–532 (Academic press, California, 2002.
Broad, J. & Sanger, G. J. The antibiotic azithromycin is a motilin receptor agonist in human stomach: comparison with erythromycin. Br. J. Pharmacol. 168, 1859–1867 (2013).
Kudoh, S. et al. Clinical effect of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis. Jpn. J. Thorac. Dis. 25, 632–642 (1987) (in Japanese with English abstract).
Kudoh, S., Azuma, A., Yamamoto, M., Izumi, T. & Ando, M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 157, 1829–1832 (1998).
Kudoh, S. et al in Macrolide Antibiotics. Chemistry, Biology, and Practice. 2nd edn, (ed. Ōmura S) Ch. 12, 533–570 (Academic press, California, 2002.
Amsden, G. W. Anti-inflammatory effects of macrolides–an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J. Antimicrob. Chemother. 55, 10–21 (2005).
Ōmura, S. et al. Gastrointestinal motor-stimulating activity of macrolide antibiotics and the structure-activity relationship. J. Antibiot. 38, 1631–1632 (1985).
Ōmura, S. et al. Macrolides with gastrointestinal motor stimulating activity. J. Med. Chem. 30, 1941–1943 (1987).
Tsuzuki, K. et al. Motilides, macrolides with gastrointestinal motor stimulating activity. I. O-Substituted and tertiary N-substituted derivatives of 8,9-anhydroerythromycin A 6,9-hemiacetal. Chem. Pharm. Bull. 37, 2687–2700 (1989).
Sunazuka, T. et al. Motilides, macrolides with gastrointestinal motor stimulating activity. II. Quaternary N-substituted derivatives of 8,9-anhydroerythromycin A 6,9-hemiacetal and 9, 9-dihydroerythromycin A 6, 9-epoxide. Chem. Pharm. Bull. 37, 2701–2709 (1989).
Li, Y. et al. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule 1 messenger RNA induction and leukocyte migration: role in preventing lung injury and fibrosis in bleomycin-challenged mice. Chest. 122, 2137–2145 (2002).
Abdelghaffar, H., Babin-Chevaye, C. & Labro, M. T. The macrolide roxithromycin impairs NADPH oxidase activation and alters translocation of its cytosolic components to the neutrophil membrane in vitro. Antimicrob. Agents Chemother. 49, 2986–2989 (2005).
Kikuchi, T. et al. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors. J. Antimicrob. Chemother. 49, 745–755 (2002).
Keicho, N., Kudoh, S., Yotsumoto, H. & Akagawa, K. S. Erythromycin promotes monocyte to macrophage differentiation. J. Antibiot. 47, 80–89 (1994).
Keicho, N., Kudoh, S., Yotsumoto, H. & Akagawa, K. S. Antilymphocytic activity of erythromycin distinct from that of FK506 or cyclosporin A. J. Antibiot. 46, 1406–1413 (1993).
Takahashi, T. et al. Erythromycin attenuates an experimental model of chronic bronchiolitis via augmenting monocyte chemoattractant protein-1. Eur. Respir. J. 17, 360–367 (2001).
Tsurita, M. et al. Early augmentation of interleukin (IL)-12 level in the airway of mice administered orally with clarithromycin or intranasally with IL-12 results in alleviation of influenza infection. J. Pharmacol. Exp. Ther. 298, 362–368 (2001).
Bosnar, M., Kelneric, Z., Munic, V., Erakovic, V. & Parnham, M. J. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob. Agents Chemother. 49, 2372–2377 (2005).
Sato, K. et al. Therapeutic effect of erythromycin on influenza virus-induced lung injury in mice. Am. J. Respir. Crit. Care Med. 157, 853–857 (1998).
Takahashi, H. et al. Roxithromycin decreases ultraviolet B irradiation-induced reactive oxygen intermediates production and apoptosis of keratinocytes. J. Dermatol. Sci. 34, 25–33 (2004).
Ueno, S. et al. Roxithromycin inhibits constitutive activation of nuclear factor κB by diminishing oxidative stress in a rat model of hepatocellular carcinoma. Clin. Cancer Res. 11, 5645–5650 (2005).
Kirst, H. A., Wind, J. A. & Paschal, J. W. Synthesis of ring-contracted derivatives of erythromycin. J. Org. Chem. 52, 4359–4362 (1987).
Yoshida, K. et al. Macrolides with promotive activity of monocyte to macrophage differentiation. J. Antibiot. 58, 79–81 (2005).
Gouda, H. et al. Three-dimensional solution structure of EM703 with potent promoting activity of monocyte-to-macrophage differentiation. Bioorg. Med. Chem. Lett. 16, 2496–2499 (2006).
Li, Y. J. et al. EM703 improves bleomycin-induced pulmonary fibrosis in mice by the inhibition of TGF-beta signaling in lung fibroblasts. Respir. Res. 7, 16 (2006).
Desaki, M. et al. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: possible role in the signaling pathway that regulates nuclear factor-kappaB activation. Antimicrob. Agents Chemother. 48, 1581–1585 (2004).
Komuro, I. et al. Erythromycin derivatives inhibit HIV-1 replication in macrophages through modulation of MAPK activity to induce small isoforms of C/EBPβ. Proc. Natl Acad. Sci. USA 105, 12509–12514 (2008).
Sugawara, A. et al. Novel 12-membered non-antibiotic macrolides from erythromycin A; EM900 series as novel leads for anti-inflammatory and/or immunomodulatory agents. Bioorg. Med. Chem. Lett. 21, 3373–3376 (2011).
Sugawara, A. et al. Novel 12-membered non-antibiotic macrolides, EM900 series with anti-inflammatory and/or immunomodulatory activity; Synthesis, structure activity-relationships and in vivo study. J. Antibiot. 65, 487–490 (2012).
Flynn, E. H., Murphy, H. W. & McMahon, R. E. Erythromycin II. Des-N-methylerythromycin and N-methyl-C14-erythromycin. J. Am. Chem. Soc. 77, 3104–3106 (1955).
Jones, P. H., Perun, T. J., Rowley, E. K. & Baker, E. J. Chemical modifications of erythromycin antibiotics. 3. Synthesis of 4” and 11 esters of erythromycin A and B. J. Med. Chem. 15, 631–634 (1972).
Raja, A., Lebbos, J. & Kirkpatrick, P. Telithromycin. Nat. Rev. Drug Discov. 3, 733–734 (2004).
Otsu, K. et al. Effects of a novel nonantibiotic macrolide, EM900, on cytokine and mucin gene expression in a human airway epithelial cell line. Pharmacology 88, 327–332 (2011).
Maderna, P. & Godson, C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochimica. et. Biophysica. Acta 1639, 141–151 (2003).
Pauwels, R. A., Buist, A. S., Calverley, P. M., Jenkins, C. R. & Hurd, S. S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am. J. Respir. Crit. Care Med. 163, 1256–1276 (2001).
Loppow, D. et al. In patients with chronic bronchitis a four week trial with inhaled steroids does not attenuate airway inflammation. Respir. Med. 95, 115–121 (2001).
Seemungal, T. A. et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 157, 1418–1422 (1998).
Nakanishi, Y. et al. Clarithromycin prevents smoke-induced emphysema in mice. Am. J. Respir. Crit. Care. Med. 179, 271–278 (2009).
Albert, R. K. et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 365, 689–698 (2011).
Wright, J. L. & Churg, A. A model of tobacco smoke-induced airflow obstruction in the guinea pig. Chest 121, 188S–191S (2002).
Martorana, P. A., Beume, R., Lucattelli, M., Wollin, L. & Lungarella, G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am. J. Respir. Crit. Care Med. 172, 848–853 (2005).
National Committee for Clinical Laboratory Standards Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals. Approved standard M31-A (NCCLS, Wayne, PA, USA, 1999.
Acknowledgements
This work was supported by a Grant for the 21st Century COE Program and a Grant-in-Aid for Scientific Research on Innovative Areas ‘Chemical Biology of Natural Products’ (24102527, 26102737 to A Sugawara). It was also financed by funds from the Quality Assurance Framework of Higher Education from The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, The Uehara Memorial Foundation (to TS), Takeda Science Foundation (to A Sugawara) and a Kitasato University Research Grant for Young Researchers (to A Sugawara). We thank Ms. Chikako Sakabe, Ms. Akiko Nakagawa and Ms. Noriko Sato (Kitasato University) for various instrumental analyses.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The paper is dedicated to Professor Amos B Smith III on the occasion of his 70th birthday.
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Sugawara, A., Shima, H., Sueki, A. et al. Non-antibiotic 12-membered macrolides: design, synthesis and biological evaluation in a cigarette-smoking model. J Antibiot 69, 319–326 (2016). https://doi.org/10.1038/ja.2015.91
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2015.91
This article is cited by
-
Microbiology testing associated with antibiotic dispensing in older community-dwelling adults
BMC Infectious Diseases (2020)