Abstract
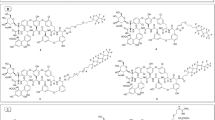
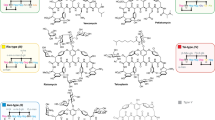
A series of lipophilic teicoplanin pseudoaglycon derivatives, including alkyl-, aryl-, calixarene- and protected sugar-containing conjugates, were prepared using azide–alkyne click chemistry. Out of the conditions applied, the CuSO4–ascorbate reagent system proved to be more efficient than the Cu(I)I–Et3N-mediated reaction. Some of the new compounds have high in vitro activity against glycopeptide-resistant Gram-positive bacteria, including vanA-positive Enterococcus faecalis. A few of them also display promising in vitro anti-influenza activity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Binda, E., Marinelli, F. & Marcone, G. L. Old and new glycopeptide antibiotics: action and resistance. Antibiotics 3, 572–594 (2014).
Van Bambeke, F., Lambert, D. M., Mingeot-Leclercq, M. & Tulkens, P. M. in Infectious Diseases 2nd edn (eds Cohen, J. & Powderly, W. G.) 1717–1720 (Mosby Publishing, London, UK, 2004).
Bager, F., Madsen, M., Christensen, J. & Aarestrup, F. M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev. Vet. Med. 31, 95–112 (1997).
Levine, D. P. Vancomycin: a history. Clin. Infect. Dis. 42, S5–S12 (2006).
Trueba, F., Garrabe, E, Hadef, R., Fabre, R. & Cavallo, J. High prevalence of teicoplanin resistance among Staphylococcus epidermidis strains in a 5-year retrospective study. J. Clin. Microbiol. 44, 1922–1923 (2006).
Sujatha, S. & Praharaj, I. Glycopeptide resistance in Gram-positive cocci: a review. Interdiscip. Perspect. Infect. Dis. 2012, 781679 (2012).
Chen, A. Y., Zervos, M. J. & Vazquez, J. A. Dalbavancin: a novel antimicrobial. Int. J. Clin. Pract. 61, 853–863 (2007).
Damodaran, S. E. & Madhan, S. Telavancin: a novel lipoglycopeptide antibiotic. J. Pharmacol. Pharmacother. 2, 135–137 (2011).
Van Bambeke, F. Renaissance of antibiotics against difficult infections: focus on oritavancin and new ketolides and quinolones. Ann. Med. 46, 512–529 (2014).
Sztaricskai, F. et al. A new series of glycopeptide antibiotics incorporating a squaric acid moiety. J. Antibiot. 59, 564–582 (2006).
Sipos, A. et al. Synthesis of isoindole and benzoisoindole derivatives of teicoplanin pseudoaglycon with remarkable antibacterial and antiviral activities. Bioorg. Med. Chem. Lett. 22, 7092–7096 (2012).
Csávás, M. et al. Synthesis and antibacterial evaluation of some teicoplanin pseudoaglycon derivatives containing alkyl- and arylthiosubstituted maleimides. J. Antibiot. 68, 579–585 (2015).
Pintér, G. et al. A diazo transfer—click reaction route to new, lipophilic teicoplanin and ristocetin aglycon derivatives with high antibacterial and anti-influenza virus activity: an aggregation and receptor binding study. J. Med. Chem. 52, 6053–6061 (2009).
Norris, E. A. & Thalia, I. N. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol. Rev. 26, 511–532 (2003).
Csávás, M. et al. Rapid synthesis of self-assembling 1,2-thiomannobioside glycoconjugates as potential multivalent ligands of mannose-binding lectins. Tetrahedron Lett. 55, 6983–6986 (2014).
Sahoo, L., Singhamahapatra, A. & Loganathan, D. Diversity oriented synthesis of novel haloglycolipids potentially useful for crystallization of integral membrane proteins. Org. Biomol. Chem. 12, 2615–2625 (2014).
Batool, T et al. A convenient method for the synthesis of (prop-2-ynyloxy)benzene derivatives via reaction with propargyl bromide. PLoS ONE 9, e115457 (2014).
Casaschi, A., Grigg, R. & Sansano, J. M. Palladium catalysed tandem cyclisation–anion capture. Part 6: Synthesis of sugar, nucleoside, purine, benzodiazepinone and β-lactam analogues via capture of in situ generated vinylstannanes. Tetrahedron 56, 7553–7560 (2000).
Tanaka, S., Goi, T., Tanaka, K. & Fukase, K. Highly efficient α-sialylation by virtue of fixed dipole effects of N-phthalyl group: Application to continuous flow synthesis of α(2-3)- and α(2-6)-Neu5Ac-Gal motifs by microreactor. J. Carbohydr. Chem. 26, 369–394 (2007).
Bois, J. et al. Easy and selective method for the synthesis of various mono-O-functionalized calix[4]arenes: de-O-functionalization using TiCl4 . J. Org. Chem. 75, 7550–7558 (2010).
Meldal, M. & Tornøe, V. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev. 108, 2952–3015 (2008).
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement. CLSI document M100-S22, Vol. 32, 1–184 (Clinical and Laboratory Standards Institute, Wayne, PA, 2012).
Gonzalez, N. et al. Influence of the MBC/MIC ratio on the antibacterial activity of vancomycin versus linezolid against methicillin-resistant Staphylococcus aureus isolates in a pharmacodynamic model simulating serum and soft tissue interstitial fluid concentrations reported in diabetic patients in a pharmacodynamic model. J. Antimicrob. Chemother. 68, 2291–2295 (2013).
Odenholt, I., Löwdin, E. & Cars, O. In vitro studies of the pharmacodynamics of teicoplanin against Staphylococcus aureus Staphylococcus epidermidis and Enterococcus faecium. Clin. Microbiol. Infect. 9, 930–970 (2003).
Chen, L. et al. Vancomycin analogues active against vanA-resistant strains inhibit bacterial transglycosylase without binding substrate. Proc. Natl Acad. Sci. USA 100, 5658–5663 (2003).
Belley, A. et al. Oritavancin disrupts membrane integrity of Staphylococcus aureus and vancomycin-resistant enterococci to effect rapid bacterial killing. Antimicrob. Agents Chemother. 54, 5369–5371 (2010).
Allen, N. E., LeTourneau, D. L. & Hobbs, J. N. The role of hydrophobic side chains as determinants of antibacterial activity of semisynthetic glycopeptide antibiotics. J. Antibiot. 50, 677–684 (1997).
Vanderlinden, E. et al. Novel inhibitors of influenza virus fusion: structure-activity relationship and interaction with the viral hemagglutinin. J.Virol. 84, 4277–4288 (2010).
Acknowledgements
We thank the financial support from the Hungarian Research Fund (OTKA K 109208). This research was also supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP-4.2.2B-15/1/KONV-2015-0001.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Szűcs, Z., Csávás, M., Rőth, E. et al. Synthesis and biological evaluation of lipophilic teicoplanin pseudoaglycon derivatives containing a substituted triazole function. J Antibiot 70, 152–157 (2017). https://doi.org/10.1038/ja.2016.80
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2016.80
This article is cited by
-
Synthesis of vancomycin fluorescent probes that retain antimicrobial activity, identify Gram-positive bacteria, and detect Gram-negative outer membrane damage
Communications Biology (2023)
-
N-Terminal guanidine derivatives of teicoplanin antibiotics strongly active against glycopeptide resistant Enterococcus faecium
The Journal of Antibiotics (2020)
-
New semisynthetic teicoplanin derivatives have comparable in vitro activity to that of oritavancin against clinical isolates of VRE
The Journal of Antibiotics (2019)
-
Fluorescence assay to predict activity of the glycopeptide antibiotics
The Journal of Antibiotics (2019)
-
Lipophilic teicoplanin pseudoaglycon derivatives are active against vancomycin- and teicoplanin-resistant enterococci
The Journal of Antibiotics (2017)