Abstract
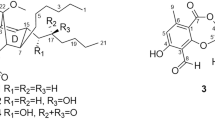
Five new pteridic acids C–G (1–5) were isolated from a culture broth of the marine-derived actinomycete Streptomyces sp. SCSGAA 0027. Their complete structures were elucidated on the basis of NMR data, modified Mosher’s method and quantum chemical calculations. Furthermore, their cytotoxicity, antiviral and antimicrobial activities were also evaluated.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sperry, J., Wilson, Z. E., Rathwellab, D. C. K. & Brimble, M. A. Isolation, biological activity and synthesis of benzannulated spiroketal natural products. Nat. Prod. Rep. 27, 1117–1137 (2010).
Bruns, N. et al. Spirangien derivatives from the myxobacterium sorangium cellulosum: isolation, structure elucidation, and biological activity. Eur. J. Org. Chem. 4, 847–857 (2015).
Sato, S., Iwata, F., Yamada, S. & Katayama, M. Neomaclafungins A–I: oligomycin-class macrolides from a marine-derived actinomycete. J. Nat. Prod. 75, 1974–1982 (2012).
Xiong, L. et al. Leonuketal, a spiroketal diterpenoid from Leonurus japonicus. Org. Lett. 17, 6238–6241 (2015).
Williams, D. E. et al. Spirastrellolide A: revised structure, progress toward the relative configuration, and inhibition of protein phosphatase 2A. Org. Lett. 6, 2607–2610 (2004).
Fremlin, L. et al. Reveromycins revealed: new polyketide spiroketals from Australian marine-derived and terrestrial Streptomyces spp. A case of natural products vs. artifacts. Org. Biomol. Chem. 9, 1201–1211 (2011).
Rho, J. R. et al. Phorbaketals A, B, and C, sesterterpenoids with a spiroketal of hydrobenzopyran moiety isolated from the marine sponge Phorbas sp. Org. Lett. 11, 5590–5593 (2009).
Frank, B. et al. Spiroketal polyketide formation in sorangium: identification and analysis of the biosynthetic gene cluster for the highly cytotoxic spirangienes. Chem. Biol. 14, 221–233 (2007).
Guérinot, A., Lepesqueux, G., Sablé, S., Reymond, S. & Cossy, J. Synthetic efforts toward the spiroketal core of spirangien A. J. Org. Chem. 75, 5151–5163 (2010).
Yadav, J. S., Reddy, N. M., Rahman, M. A. & Prasad, A. R. Synthesis of the spiroketal fragment of (–)-ushikulide. Eur. J. Org. Chem. 25, 5574–5581 (2014).
Butler, B. B., Manda, J. N. & Aponick, A. Synthesis of the spirastrellolide A, B/C spiroketal: enabling solutions for problematic Au(I)-catalyzed spiroketalizations. Org. Lett. 17, 1902–1905 (2015).
Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H. G. & Prinsep, M. R. Marine natural products. Nat. Prod. Rep. 33, 382–431 (2016).
Vinothkumar, S. & Parameswaran, P. S. Recent advances in marine drug research. Biotechnol. Adv. 31, 1826–1845 (2013).
Nong, X. H., Zhang, X. Y., Xu, X. Y., Wang, J. & Qi, S. H. Nahuoic acids B–E, polyhydroxy polyketides from the marine-derived Streptomyces sp. SCSGAA 0027. J. Nat. Prod. 79, 141–148 (2016).
Igarashi, Y., Iida, T., Yoshida, R. & Furumai, T. Pteridic acids A and B, novel plant growth promoters with auxin-like activity from Streptomyces hygroscopicus TP-A0451. J. Antibiot. 55, 764–767 (2002).
Řezanka, T., Dvořáková, R., Hanuš, L. O. & Dembitsky, V. P. Enteridinines A and B from slime mold Enteridium lycoperdon. Phytochemistry 65, 455–462 (2004).
Wagenaar, M. M., Williamson, R. T., Ho, D. M. & Carter, G. T. Structure and absolute stereochemistry of 21-hydroxyoligomycin A. J. Nat. Prod. 70, 367–371 (2007).
Chen, Y. P. et al. Streptospirodienoic acids A and B, 6,6-spiroketal polyketides from Streptomyces sp. RSC Adv. 4, 63324–63327 (2014).
Acar, J. F., Goldstein, F. W., Kitzis, M. D. & Eyquem, M. T. Resistance pattern of anaerobic bacteria isolated in a general hospital during a two-year period. J. Antimicrob. Chemother. 8, 9–16 (1981).
Pauwels, R. et al. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20, 309–321 (1988).
Ma, F. et al. Anti-HSV activity of kuwanon X from mulberry leaves with genes expression inhibitory and HSV-1 induced NF-κB deactivated properties. Biol. Pharm. Bull. 39, 1667–1674 (2016).
Frisch, M. J. et al Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford, CT, USA, (2010).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Yanai, T., Tew, D. P. & Handy, N. C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004).
Schäfer, A., Huber, C. & Ahlrichs, R. J. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. Chem. Phys. 100, 5829–5835 (1994).
Bruhn, T., Schaumlöffel, A., Hemberger, Y. & Bringmann, G. SpecDis: quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 25, 243–249 (2013).
Acknowledgements
We are grateful for the financial support provided by the National Natural Science Foundation of China (41376160 and 81673326), National Natural Science Foundation of China (41606100), Strategic Leading Special Science and Technology Program of Chinese Academy of Sciences (XDA100304002), Natural Science Foundation of Guangdong Province, China (2015A030310349) and Regional Innovation Demonstration Project of Guangdong Province Marine Economic Development (GD2012-D01-002). We appreciate the contribution of the analytical facility center (Xiao Zhihui, Li Chuanrong, Sun Aijun and Zhang Yun) of South China Sea Institute of Oceanology, Chinese Academy of Sciences for acquiring NMR, HRESIMS and experimental CD data. We are also grateful for the support given by the Guangzhou Branch of the Supercomputing Center of Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Nong, XH., Wei, XY. & Qi, SH. Pteridic acids C–G spirocyclic polyketides from the marine-derived Streptomyces sp. SCSGAA 0027. J Antibiot 70, 1047–1052 (2017). https://doi.org/10.1038/ja.2017.105
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2017.105
This article is cited by
-
Development of a drug discovery approach from microbes with a special focus on isolation sources and taxonomy
The Journal of Antibiotics (2023)
-
Iseoic acids and bisiseoate: three new naphthohydroquinone/naphthoquinone-class metabolites from a coral-derived Streptomyces
The Journal of Antibiotics (2023)
-
Streptomyces alleviate abiotic stress in plant by producing pteridic acids
Nature Communications (2023)
-
Isolation and structure determination of allopteridic acids A–C and allokutzmicin from an unexplored actinomycete of the genus Allokutzneria
The Journal of Antibiotics (2023)