Abstract
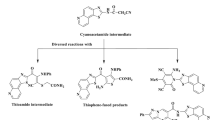
In order to modify lincomycin at the C-6 and C-7 positions, we prepared target molecules, which have substituted pipecolinic acid at the 6-amino group and a para-substituted phenylthio group at the C-7 position, in application of palladium-catalyzed cross-coupling as a key reaction. As the result of structure-activity relationship (SAR) studies at the 6-position, analogs possessing 4′-cis-(cyclopropylmethyl)piperidine showed significantly strong antibacterial activities against Streptococcus pneumoniae and Streptococcus pyogenes with an erm gene. On the basis of SAR, we further synthesized novel analogs possessing 4′-cis-(cyclopropylmethyl)piperidine by transformation of a C-7 substituent. Consequently, novel derivatives possessing a para-heteroaromatic-phenylthio group at the C-7 position exhibited significantly strong activities against S. pneumoniae and S. pyogenes with an erm gene even when compared with those of telithromycin. Finally, in vivo efficacy of selected two derivatives was evaluated in a rat pulmonary infection model with resistant S. pneumoniae with erm + mef genes. One of them exhibited strong and constant in vivo efficacy in this model, and both compounds showed strong in vivo efficacy against resistant S. pneumoniae with a mef gene.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Reinert, R. R ., van der Linden, M. & Al-Lahham, A. Molecular characterization of the first telithromycin-resistant Streptococcus pneumoniae isolate in Germany. Antimicrob. Agents Chemother. 49, 3520–3522 (2005).
Kim, S. H. et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob. Agents Chemother. 56, 1418–1426 (2012).
Ajito, K ., Miura, T ., Furuuchi, T. & Tamura, A. Sixteen-membered macrolides: chemical modifications and future applications. Heterocycles 89, 281–352 (2014).
Morimoto, S ., Takahashi, Y ., Watanabe, Y. & Omura, S. Chemical modification of erythromycins I. Synthesis and antibacterial activity of 6-O-methylerythromycins A. J. Antibiot. 37, 187–189 (1984).
Slobodan, D. et al. Erythromycin series. Part 13. Synthesis and structure elucidation of 10-dihydro-10-deoxo-11-methyl-11-azaerythromycin A. J. Chem. Res. Synop. 40, 152–153 (1988).
Retsema, J. et al. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against Gram-Negative Organisms. Antimicrob. Agents Chemother. 31, 1939–1947 (1987).
Denis, A. et al. Synthesis and antibacterial activity of HMR 3647 a new ketolide highly potent against erythromycin-resistant and susceptible pathogens. Bioorg. Med. Chem. Lett. 9, 3075–3080 (1999).
Clay, K. D. et al. Severe hepatotoxicity of telithromycin: three case reports and literature review. Ann. Intern. Med. 144, 415–420 (2006).
Ross, D. B. The FDA and the case of Ketek. N. Engl. J. Med. 356, 1601–1604 (2007).
Gleason, P. P ., Walters, C ., Heaton, A. H. & Schafer, J. A. Telithromycin: the perils of hasty adoption and persistence of off-label prescribing. J. Manag. Care Pharm. 13, 20–25 (2007).
Department of Health and Human Services. Telithromycin (marketed as Ketek) information available at http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm107824.htm Accessed 26 April 2007.
Miura, T. et al. Novel azalides derived from sixteen-membered macrolides. I. Isolation of the mobile dialdehyde and its one-pot macrocyclization with an amine. J. Antibiot. 60, 407–435 (2007).
Miura, T. et al. Novel azalides derived from 16-membered macrolides. III. Azalides modified at the C-15 and 4” positions: improved antibacterial activities. Bioorg. Med. Chem. 18, 2735–2747 (2010).
Mason, D. J ., Dietz, A. & Deboer, C. Lincomycin, a new antibiotic I. Discovery and biological properties. Antimicrob. Agents Chemother. 554–559 (1962).
Magerlein, B. J. & Lincomycin, X. The chemical synthesis of lincomycin. Tetrahedron Lett. 1, 33–36 (1970).
Howarth, G. B ., Szarek, W. A. & Jones, J. K.N. The synthesis of lincomycin. J. Chem. Soc. (c) 16, 2218–2224 (1970).
Perlman, D. Structure-Activity Relationships Among the Semisynthetic Antibiotics. Academic Press: New York, San Francisco, London, A Subsidiary of Harcourt Brace Jovanovich Publishers, 1977, pp 600–651.
Birkenmeyer, R. D. & Kagan, F. Lincomycin. XI. Synthesis and structure of clindamycin. A potent antibacterial agent. J. Med. Chem. 13, 616–619 (1970).
Shan, P. J ., Vakil, N. & Kabakov, A. Role of intravenous immune globulin in streptococcal toxic shock syndrome and Clostridium difficile infection. Am. J. Health Syst. Pharm. 72, 1013–1019 (2015).
Hoeksema, H. Octoses from antibiotics. The Upjohn Company, Kalamazoo, Mich.,Abstr. Pap. Division of Carbohydrate Chemistry, 149th Meet, American Chemical Society. Am. Chem Soc. Detroit, Mich p 9C (1965).
Magerlein, B. J ., Birkenmeyer, R. D. & Kagan, F. Chemical modification of lincomycin. Antimicrob. Agents Chemother. 727–736 (1966).
Sinkula, A. A ., Morozowich, W ., Lewis, C. & Mackellar, F. A. Synthesis and bioactivity of lincomycin-7-monoesters. J. Pharm. Sci. 58, 1389–1392 (1969).
Magerlein, B. J. & Kagan, F. Lincomycin. IX. 7-Thiol and thioamido analogs of lincomycin. J. Med. Chem. 12, 974–977 (1969).
Lewis, J. G. et al Novel Antimicrobial 7-methyl Lincosamides: Prolamide Analogs. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy Poster F-1388; Washington, DC, USA.
Bannister, B. Modifications of lincomycin involving the carbohydrate portion. Part III. The 7-O-methyl and 6-de-(1-hydroxyethyl) analogues. J. Chem. Soc. Perkin Trans. I 1676–1682 (1973).
Bannister, B. Modifications of lincomycin involving the carbohydrate portion. Part IV. (7S-7-alkoxy-7-deoxy-analogues. J. Chem. Soc. Perkin Trans. I 1974, 360–369 (1974).
Bannister, B. & Mydlow, P. K. The S-alkylation of sulphides by an activated carbohydrate epimine under acidic catalysis: the formation of α-acetamido-sulphides. Part 5. The introduction of functionality into the sulphide substituent. J. Chem. Res. (S) 1989, 90–91 (1989).
Bannister, B. The S-alkylation of sulphides by an activated carbohydrate epimine under acidic catalysis: The formation of α-acetamido-sulphides. Part 4. Reaction with dithioacetals and monothioacetals. J. Chem. Soc. Perkin. Trans. I 1980, 540–552 (1980).
Bannister, B. 7S-7-deoxy-7-substituted-alkylthio-lincomycin. S-Alkylation of sulphides by an activated epimine under acidic catalysis: formation of α-acetamido-sulphides. Tetrahedron 40, 1633–1660 (1984).
Bannister, B. The Upjohn Company. Derivatives of lincomycin and its analogs and process. US Patent US3915954 A (1973).
Bannister, B. The Upjohn Company. Derivatives of lincomycin and its analogs and process. Canadian Patent CA-971956 A1 (1972).
Sztaricskai, F. et al. Semisynthetic modification of antibiotic lincomycin. J. Antibiot. 49, 941–943 (1996).
Umemura, E. et al Lincomycin derivative and antibacterial agent containing the same as active ingredient. Japanese Patent WO/2007/066805 A1 (14 June 2007).
Wakiyama, Y. et al Lincomycin derivatives and antibacterial agents containing the same as the active ingredient. Japanese Patent WO/2008/146917 A1 (4 December 2008).
Umemura, E. et al Lincosamide derivative, and antibacterial agent comprising the same as active ingredient, WO/2008/146919 A1 (4 December 2008).
Umemura, E. et al. Synthesis of novel lincomycin derivatives and their in vitro antibacterial activities. J. Antibiot. 66, 195–198 (2013).
Wakiyama, Y. et al. Synthesis and structure–activity relationships of novel lincomycin derivatives. Part 1. Newly generated antibacterial activities against Gram-positive bacteria with erm gene by C-7 modification. J. Antibiot. 69, 368–380 (2016).
Wakiyama, Y. et al. Synthesis and structure–activity relationships of novel lincomycin derivatives. Part 2. Synthesis of 7(S)-7-deoxy-7-(4-morpholinocarbonylphenylthio)lincomycin and its 3-dimensional analysis with rRNA. J. Antibiot. 69, 428–439 (2016).
Kumura, K. et al. Synthesis and antibacterial activity of novel lincomycin derivatives. I. Enhancement of antibacterial activities by introduction of substituted azetidines. J. Antibiot. 69, 440–445 (2016).
Wakiyama, Y. et al. Synthesis and structure–activity relationships of novel lincomycin derivatives Part 3: discovery of the 4-(pyrimidin-5-yl)phenyl group in synthesis of 7(S-thiolincomycin analogs. J. Antibiot. 70, 52–64 (2017).
Kumura, K. et al. Synthesis and antibacterial activity of novel lincomycin derivatives. II. Exploring (7S-7-(5-aryl-1,3,4-thiadiazol-2-yl-thio)-7-deoxylincomycin derivatives. J. Antibiot. 70, 655–663 (2017).
Wakiyama, Y. et al. Synthesis and structure-activity relationships of novel lincomycin derivatives Part 4. Synthesis of novel lincomycin analogs modified at the 6- and 7-positions and their potent antibacterial activities. J. Antibiot. 70, 888–906 (2017).
Kumura, K. et al Synthesis and antibacterial activity of novel lincomycin derivatives. III. Optimization of a phenyl thiadiazole moiety. J. Antibiot. (e-pub ahead of print 5 July 2017; doi:10.1038/ja.2017.59.
Argoudelis, A. D ., Coats, J. H ., Mason, D. J. & Sebek, O. K. Microbial transformation of antibiotics. III. Conversion of clindamycin to 1′-demethylclindamycin and clindamycin sulfoxide by Streptomyces species. J. Antibiot. 22, 309–314 (1969).
Magerlein, B. J ., Birkenmeyer, R. D. & Kagan, F. Lincomycin. VI. 4′-alkyl analogs of lincomycin. Relationship between structure and antibacterial activity. J. Med. Chem. 10, 355–359 (1967).
Magerlein, B. J. Lincomycin. 14. An improved synthesis and resolution of the antimalarial agent, 1′-demethyl-4′-depropyl-4′ (R- and -(S-pentylclindamycin hydrochloride (U-24,729A). J. Med. Chem. 15, 1255–1259 (1972).
Magerlein, B. J. & Lincomycin. VII. 4′-depropyl-4′-ethoxylincomycins. J. Med. Chem. 10, 1161–1163 (1967).
Lewis, J. G. et al Novel lincomycin derivatives possessing antimicrobial activity. WO/2006/055070 A2 (26 May 2006).
Birkenmeyer, R. D ., Kroll, S. J ., Lewis, C ., Stern, K. F. & Zurenko, G. E. Synthesis and antimicrobial activity of clindamycin analogues: pirlimycin, a potent antibacterial agent. J. Med. Chem. 27, 216–223 (1984).
O’Dowd, H. et al. Novel antibacterial azetidine lincosamides. Bioorg. Med. Chem. 18, 2645–2648 (2008).
O’Dowd, H. et al Novel antibacterial azetidine lincosamides. 44 the Interscience Conference on Antimicrobial Agents and Chemotherapy. Poster F-2037; Washington, DC, USA, 2004.
Lewis, J. G. et al Novel antimicrobial 7-methyl lincosamides: Pipecolamide analogs. 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Poster F-1389; Washington, DC, USA, 2004.
Chen, T. et al Novel 4′-cycloalkyl pipecolamide lincosamide analogs. 44 th Interscience Conference on Antimicrobial Agents and Chemotherapy. Poster F-2036; Washington, DC, USA, 2004.
Lopez, S. L. et al Characterization of the spectrum of in vitro activity of VIC-105555, a new lincosamide. 44 th Interscience Conference on Antimicrobial Agents and Chemotherapy. Poster F-2038; Washington, DC, USA, 2004.
Shuman, R. T ., Ornstein, P. L ., Paschal, J. W . & Gesellchen, P. D . An improved synthesis of homoproline and derivatives. J. Org. Chem. 55, 738–741 (1990).
Schroeder, W ., Bannister, B. & Hoeksema, H. Lincomycin. III. The structure and stereochemistry of the carbohydrate moiety. J. Am. Chem. Soc. 89, 2448–2453 (1967).
Houtman, R. L. & Mich, P. The Upjohn Company. Trimethylsilyl ethers of lincomycin and its compounds. US Patent US3418414 (1966).
Itoh, T. & Mase, T. A general palladium-catalyzed coupling of aryl bromides/triflates and thiols. Org. Lett 6, 4587–4590 (2004).
Magerlein, B. J. & Kagan, F. Lincomycin. 8. 4′-Alkyl-1′-demethyl-4′-depropylclindamycins, potent antibacterial and antimalarial agents. J. Med. Chem. 12, 780 (1969).
Farrell, D. J ., Morrissey, I ., Bakker, S. & Felmingham, D. Molecular characterization of macrolide resistance mechanisms among Streptococcus pneumoniae and Streptococcus pyogenes isolated from the PROTEKT 1999-2000 study. J. Antimicrob. Chemother. 50 (suppl_2), 39–47 (2002).
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement. CLSI Document M100-S16, Clinical and Laboratory Standards Institute, Wayne, PA, (2006).
Acknowledgements
We thank Mr A. Tamura, Dr E. Shitara and Dr T. Yoshida for encouragement and valuable discussion. We are grateful to Professor Emeritus Dr M. Konno for supervision through our in-house drug discovery program in LCM field. We also thank Dr T. Murata for ROESY analysis; Ms M. Ishii for direction in intellectual properties; Mr T. Watanabe for computational chemistry; Ms T. Miyara, Ms S. Miki and Ms K. Kaneda for analytical and synthetic chemistry; Mr Y. Takayama and Ms K. Yamada for biological studies; and Ms M. Takagi and Ms Y. Saito for English manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Wakiyama, Y., Kumura, K., Umemura, E. et al. Synthesis and SARs of novel lincomycin derivatives Part 5: optimization of lincomycin analogs exhibiting potent antibacterial activities by chemical modification at the 6- and 7-positions. J Antibiot 71, 298–317 (2018). https://doi.org/10.1038/ja.2017.114
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/ja.2017.114
This article is cited by
-
Characterization of compound A, a novel lincomycin derivative active against methicillin-resistant Staphylococcus aureus
The Journal of Antibiotics (2021)