Abstract
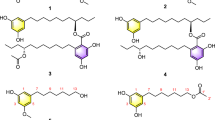
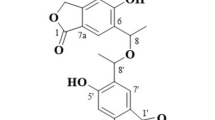
The novel compound, 11-O-methylpetasitol (1), was isolated from Penicillium sp. N-175-1, and two new compounds, cosmochlorins D (5) and E (6), were isolated from Phomopsis sp. N-125. In addition, three known eremophilane sesquiterpenes, sporogen-AO1 (2), petasol (3) and 6-dehydropetasol (4), were isolated from Penicillium sp. N-175-1. The structures of 1, 5 and 6 were elucidated by a combination of extensive spectroscopic analyses, including 2D NMR, high-resolution electrospray ionization time-of-flight mass spectrometry (HRESITOFMS) and chemical reactions. Compounds 2, 3, 5 and 6 exhibited cytotoxicity to HL60 and 2 and 3 to HeLa cells. Furthermore, 2 and 3 showed robust growth-restoring activity of a Saccharomyces cerevisiae (cdc2-1 rad9Δ) mutant strain, whereas 5 and 6 exhibited minor growth-restoring activity in this strain. Thus, these compounds may inhibit the growth of HL60 and HeLa cells by blocking the cell cycle, and they may be utilized as new lead compounds that act as inhibitors of survival signal transduction pathways.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Deng, Z. & Cao, L. Fungal endophytes and their interactions with plants in phytoremediation. Chemosphere 168, 1100–1106 (2017).
Kusari, S., Hertweck, C. & Spiteller, M. Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem. Biol. 19, 792–798 (2012).
Patil, R. H., Patil, M. P. & Maheshwari, V. L. Bioactive secondary metabolites from endophytic fungi: a review of biotechnological production and their potential applications. Stud. Nat. Prod. Chem. 49, 189–205 (2016).
Tirilly, Y., Kloosterman, J., Sipma, G. & Kettenes-Van Den Bosch, J. J. A fungitoxic sesquiterpene from Hansfordia pulvinata. Phytochemistry 22, 2082–2083 (1983).
Sugama, K., Hayashi, K., Nakagawa, T., Mitsuhashi, H. & Yoshida, N. Sesquiterpenoids from Petasites fragrans. Phytochemistry 22, 1619–1622 (1983).
Sugawara, F. et al. Phytoactive eremophilanes produced by the weed pathogen Drechslera gigantea. Biosci. Biotechnol. Biochem. 57, 236–239 (1993).
Ohtani, I., Kusumi, T., Kashman, H. & Kakisawa, H. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 113, 4092–4096 (1991).
Naya, K. & Takagi, I. The structure of petasitin, a new sesquiterpene from Petasites japonicus Maxim. Tetrahed. Lett. 5, 629–632 (1968).
Takagi, I., Tazuke, Y. & Naya, K. The components of Petasites japonicus Maxim. IV. The structure of petasitin. Bull. Chem. Soc. Jpn 50, 3320–3323 (1977).
Yan, X. H., Gavagnin, M., Cimino, G. & Guo, Y. W. Two new biscembranes with unprecedented carbon skeleton and their probable biogenetic precursor from the Hainan soft coral Sarcophyton latum. Tetrahed. Lett. 48, 5313–5316 (2007).
Motohashi, K. et al. New sesquiterpenes, JBIR-27 and -28, isolated from a tunicate-derived fungus, Penicillium sp. SS080624SCf1. J. Antibiot. 62, 247–250 (2009).
Hatakeyama, T., Koseki, T., Murayama, T. & Shiono, Y. Eremophilane sesquiterpenes from the endophyte Microdiplodia sp. KS 75-1 and revision of the stereochemistries of phomadecalins C and D. Phytochem. Lett. 3, 148–151 (2010).
Le, D. H., Takenaka, Y, Hamada, N. & Tanahashi, T. Eremophilane-type sesquiterpenes from cultured lichen mycobionts of Sarcographa tricosa. Phytochemistry 91, 242–248 (2013).
Huang, Y. F., Qiao, L., Li, Lv,A., Peib, Y. H. & Tian, L. Eremophilane sesquiterenes from the marine fungus Penicillium sp. BL27-2. Chin. Chem. Lett. 19, 562–564 (2008).
Singh, S. B. et al. Structure and absolute stereochemistry of HIV-1 integrase inhibitor integric acid. A novel eremophilane sesquiterpenoid produced by a Xylaria sp. Tetrahed. Lett. 40, 8775–8779 (1999).
Ogasawara, Y., Yoshida, J., Shiono, Y., Miyakawa, T. & Kimura, K. New eremophilane sesquiterpenoid compounds, eremoxylarins A and B directly inhibit calcineurin in a manner independent of immunophilin. J. Antibiot. 61, 496–502 (2008).
Shiono, Y. et al. GSK-3β inhibitory activities of novel dichlororesorcinol derivatives from Cosmospora vilior isolated from a mangrove plant. Phytochem. Lett. 18, 122–127 (2016).
Tsuchiya, E., Yukawa, M., Miyakawa, T., Kimura., K. & Takahashi, H. Borrelidin inhibits a cyclin-dependent kinase (CDK), Cdc28/Cln2, of Saccharomyces cerevisiae. J. Antibiot. 54, 84–90 (2010).
Tsuchiya, E., Yukawa, M., Ueno, M., Kimura, K. & Takahashi, H. A novel method of screening cell-cycle blockers as candidates for anti-tumor reagents using yeast as a screening tool. Biosci. Biotechnol. Biochem. 74, 411–414 (2010).
Kimura, K. et al. Cleavage mechanism and anti-tumor activity of 3,6-epidioxy-1,10-bisaboladiene isolated from edible wild plants. Bioorg. Med. Chem. 20, 3887–3897 (2012).
Acknowledgements
We thank Emeritus Professor Eiko Tsuchiya of Hiroshima University for giving us a mutant strain, Saccharomyces cerevisiae (cdc2-1 rad9Δ), and Japan Student Services Organization (JASSO, the Student Exchange Support Program) for the scholarship to NIM. This work was partly supported by MEXT KAKENHI Grant Number JP15H05246.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Shiono, Y., Muslihah, N., Suzuki, T. et al. New eremophilane and dichlororesorcinol derivatives produced by endophytes isolated from Ficus ampelas. J Antibiot 70, 1133–1137 (2017). https://doi.org/10.1038/ja.2017.125
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2017.125
This article is cited by
-
Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi
Fungal Diversity (2021)
-
Endophytic Penicillium species and their agricultural, biotechnological, and pharmaceutical applications
3 Biotech (2020)