Abstract
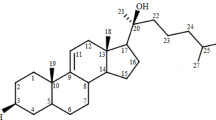
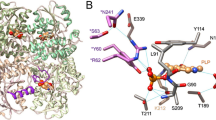
Two new analogs of halistanol sulfate (1) were isolated from a marine sponge Halichondria sp. collected at Hachijo-jima Island. Structures of these new halistanol sulfates I (2) and J (3) were elucidated by spectral analyses. Compounds 1–3 showed inhibitory activity against SIRT 1-3 with IC50 ranges of 45.9–67.9, 18.9–21.1 and 21.8-37.5 μM, respectively. X-ray crystallography of the halistanol sulfate (1) and SIRT3 complex clearly indicates that 1 binds to the exosite of SIRT3 that we have discovered in this study.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Correia, M. et al. Sirtuins in metabolism, stemness and differentiation. Biochim. Biophys. Acta 1861, 3444–3455 (2017).
Hall, J. A., Dominy, J. E., Lee, Y. & Puigserver, P. The sirtuin family’s role in aging and age-associated pathologies. J. Clin. Invest. 123, 973–979 (2013).
Narayan, N. et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature 492, 199–204 (2012).
Chopra, V. et al. The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington’s disease mouse models. Cell Rep. 2, 1492–1497 (2012).
Fusetani, N. & Matsunaga, S. Bioactive marine metabolites. II. Halistanol sulfate, an antimicrobial novel steroid sulfate from the marine sponge Halichondria cf. moorei Bergquist. Tetrahedron Lett. 22, 1985–1988 (1981).
Slate, D., Lee, R. H., Rogoriguez, J. & Crews, P. The marine natural product, halistanol trisulfate, inhibits pp60v-src protein tyrosine kinase activity. Biochem. Biophys. Res. Commun. 203, 260–264 (1994).
Lima, B. et al. Halistanol sulfate A and rodriguesines A and B are antimicrobial and antibiofilm agents against the cariogenic bacterium Streptococcus mutans. Rev. Bras. Farmacogn. 24, 651–659 (2014).
Cardellina, J. H. II et al. A chemical screening strategy for the dereplication and prioritization of HIV-inhibitory aqueous natural products extracts. J. Nat. Prod. 56, 1123–1129 (1993).
McKee, T. C., Cardellina, J. H. II, Tischler, M., Snader, K. M. & Boyd, M. R. Ibisterol sulfate, a novel HIV-inhibitory sulfated sterol from the deep water sponge Topsentia sp. Tetrahedron Lett. 34, 389–392 (1993).
McKee, T. C. et al. HIV-inhibitory natural products. 11. Comparative studies of sulfated sterols from marine invertebrates. J. Med. Chem. 37, 793–797 (1994).
Nakao, Y. & Fusetani, N. Enzyme inhibitors from marine invertebrates. J. Nat. Prod. 70, 689–710 (2007).
Inada, A. et al. Unusual cyclolanostanes from leaves of Pandanus boninensis. Phytochemistry 66, 2729–2733 (2005).
Sun, H. H., Cross, S. S., Gunasekera, M. & Koehn, F. E. Weinbersterol disulfates A and B, antiviral steroid sulfates from the sponge Petrosia weinbergi. Tetrahedron 47, 1185–1190 (1991).
Bifulco, G., Bruno, I., Minale, L. & Riccio, R. Novel HIV-inhibitory halistanol sulfates F-H from a marine sponge Pseudoaxinissa digitata. J. Nat. Prod. 57, 164–167 (1994).
Tsukamoto, S., Kato, H., Hirota, H. & Fusetani, N. Antifouling terpenes and steroids against barnacle larvae from marine sponges. Biofouling 11, 283–291 (1997).
Rudi, A. et al. Clathsterol, a novel anti-HIV-1 RTase sulphated sterol from the sponge Clathria species. J. Nat. Prod. 64, 1451–1453 (2001).
Queresi, A & Faulkner, D. J Haplosamates A and B: new steroidal sulfamate esters from two haplosclerida sponges. Tetrahedron 55, 8323–8330 (1999).
Jin, L. et al. Crystal structures of human SIRT3 displaying substrate-induced conformational changes. J. Biol. Chem. 284, 24394–24405 (2009).
Jin, L. et al. The anticoagulant activation of antithrombin by heparin. Proc. Natl Acad. Sci. USA 94, 14683–14688 (1997).
Nguyen, G. T. T., Gertz, M. & Steegborn, C. Crystal structures of Sirt3 complexes with 4'-bromo-resveratrol reveal binding sites and inhibition mechanism. Chem. Biol. 20, 1375–1385 (2013).
Kupchan, S. M., Briton, R. W., Ziegler, M. F. & Sigel, C. W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 38, 178–179 (1973).
Shah, A. A., Ito, A., Nakata, A. & Yoshida, M. Identification of a selective SIRT2 inhibitor and its anti-breast cancer activity. Biol. Pharm. Bull. 39, 1739–1742 (2016).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Macromol. Crystallogr. A 276, 307–326 (1997).
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Cryst. 30, 1022–1025 (1997).
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Acknowledgements
This paper is a part of the outcome of research performed under a Waseda University Grant-in-Aid for Scientific Research, the Strategic Research Platforms for Private University, a Matching Fund Subsidy from the Ministry of Education, Science, Sports, and Technology (MEXT), Japan, JSPS KAKENHI Grant Number 26221204 and the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct). This work was inspired by the JSPS Asian Chemical Biology Initiative. This work was performed under the approval of the Photon Factory Program Advisory Committee (Proposal Nos. 2013G674 and 2015G615). Atomic coordinates and structural factors have been deposited in the Protein Data Bank (PDB id 3GLU).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Nakamura, F., Kudo, N., Tomachi, Y. et al. Halistanol sulfates I and J, new SIRT1–3 inhibitory steroid sulfates from a marine sponge of the genus Halichondria. J Antibiot 71, 273–278 (2018). https://doi.org/10.1038/ja.2017.145
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2017.145