Abstract
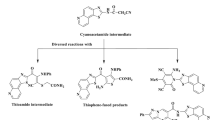
Molecular hybridization approach is an emerging tool in drug discovery for designing new pharmacophores with biological activity. A novel, new series of coumarin–benzimidazole hybrids were designed, synthesized and evaluated for their broad spectrum antimicrobial activity. Among all the synthesized molecules, compound (E)-3-(2-1H-benzo[d]imidazol-1-yl)-1-((4-chlorobenzyl)oxy)imino)ethyl)-2H-chromen-2-one showed the most promising broad spectrum antibacterial activity against Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis and Proteus vulgaris. In addition, it has showed no cytotoxicity and hemolysis at 10 times the MIC concentration. SAR studies indicate that position of the chlorine atom in the hybrid critically determines the antibacterial activity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Khan, S. N. & Khan, A. U. Breaking the spell: combating multidrug resistant 'Superbugs'. Front. Microbiol. 7, 174 (2016).
Tommasi, R., Brown, D. G., Walkup, G. K., Manchester, J. I. & Miller, A. A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 14, 529–542 (2015).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661 (2016).
Vuorelaa, P. et al. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 11, 1375–1389 (2004).
Appendino, G. et al. Antimycobacterial coumarins from the sardinian giant fennel (Ferula communis). J. Nat. Prod. 67, 2108–2110 (2004).
Binda, C. et al. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. J. Med. Chem. 50, 5848–5852 (2007).
Kontogiorgis, C. A. & Hadjipavlou-Litina, D. J. Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg. Med. Chem. Lett. 14, 611–614 (2004).
Ma, T. et al. Chemical library and structure-activity relationships of 11-demethyl-12-oxo calanolide A analogues as anti-HIV-1 agents. J. Med. Chem. 51, 1432–1446 (2008).
Sashidhara, K. V. et al. Molecular iodine catalysed one-pot synthesis of chromeno[4,3-b]quinolin-6-ones under microwave irradiation. Green Chem. 17, 3766–3770 (2015).
Shi, Y. & Zhou, C. H. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 21, 956–960 (2011).
Tambo-ong, A., Chopra, S., Glaser, B. T., Matsuyama, K., Tran, T. & Madrid, P. B. Mannich reaction derivatives of novobiocin with modulated physiochemical properties and their antibacterial activities. Bioorg. Med. Chem. Lett. 21, 5697–5700 (2011).
Laurin, P. et al. Synthesis and in vitroevaluation of novel highly potent coumarin inhibitors of gyrase B. Bioorg. Med. Chem. Lett. 9, 2079–2084 (1999).
Schimana, J. et al. Simocyclinones, novel cytostatic angucyclinone antibiotics produced by Streptomyces antibioticus Tu 6040. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 53, 779–787 (2000).
Watt, P. M. & Hickson, I. D. Structure and function of type II DNA topoisomerases. Biochem. J. 303 (Pt 3), 681–695 (1994).
Li, B. et al. Coumarin-based inhibitors of Bacillus anthracis and Staphylococcus aureus replicative DNA helicase: chemical optimization, biological evaluation, and antibacterial activities. J. Med. Chem. 55, 10896–10908 (2012).
Sahoo, J. & Paidesetty, S. K. Antimicrobial activity of novel synthesized coumarin based transitional metal complexes. J. Taibah Univ. Med. Sci. 12, 115–124 (2017).
Paul, K., Bindal, S. & Luxami, V. Synthesis of new conjugated coumarin-benzimidazole hybrids and their anticancer activity. Bioorg. Med. Chem. Lett. 23, 3667–3672 (2013).
Al-Majedy, Y. K., Kadhum, A. A. H., Al-Amiery, A. A. & Abu Bakar Mohamad, A. B. Coumarins: the antimicrobial agents. Sys. Rev. Pharm. 8, 62–70 (2017).
Sashidhara, K. V. et al. Designing, synthesis of selective and high-affinity chalcone-benzothiazole hybrids as Brugia malayi thymidylate kinase inhibitors: in vitro validation and docking studies. Eur. J. Med. Chem. 103, 418–428 (2015).
Sashidhara, K. V. et al. Design, synthesis and anticancer activity of dihydropyrimidinone-semicarbazone hybrids as potential human DNA ligase 1 inhibitors. Med. Chem. Commun. 7, 2349–2363 (2016).
Viegas-Junior, C., Danuello, A., da Silva Bolzani, V., Barreiro, E. J. & Fraga, C. A. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 14, 1829–1852 (2007).
Narasimhan, B., Sharma, D. & Kumar, P. Benzimidazole: a medicinally important heterocyclic moiety. Med. Chem. Res. 21, 269–283 (2012).
Zhang, D. et al.. Design, synthesis and antibacterial activity of novel actinonin derivatives containing benzimidazole heterocycles. Eur. J. Med. Chem. 44, 2202–2210 (2009).
Walsh, J. J. & Bell, A. Hybrid drugs for malaria. Curr. Pharm. Des. 15, 2970–2985 (2009).
Teiten, M. H., Dicato, M. & Diederich, M. Hybrid curcumin compounds: a new strategy for cancer treatment. Molecules 19, 20839–20863 (2014).
Arora, R. K., Kaur, N., Bansal, Y. & Bansal, G. Novel coumarin-benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm. Sin. B 4, 368–375 (2014).
Holiyachi, M. et al. Design, synthesis and structure-activity relationship study of coumarin benzimidazole hybrid as potent antibacterial and anticancer agents. ChemistrySelect 1, 4638–4644 (2016).
Vijesh, A. M., Isloor, A. M., Prabhu, V., Ahmad, S. & Malladi, S. Synthesis, characterization and anti-microbial studies of some novel 2,4-disubstituted thiazoles. Eur. J. Med. Chem. 45, 5460–5464 (2010).
Soltani Rad, M. N., Khalafi-Nezhad, A. & Behrouz, S. Design and synthesis of some novel oxiconazole-like carboacyclic nucleoside analogues, as potential chemotherapeutic agents. Helv. Chim. Acta 92, 1760–1774 (2009).
Hanel, H. & Raether, W. A more sophisticated method of determining the fungicidal effect of water-insoluble preparations with a cell harvester, using miconazole as an example. Mycoses 31, 148–154 (1988).
Hernandes, M. Z., Cavalcanti, S. M., Moreira, D. R., de Azevedo Junior, W. F. & Leite, A. C. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr. Drug Targets 11, 303–314 (2010).
Liaras, K., Geronikaki, A., Glamoclija, J., Ciric, A. & Sokovic, M. Thiazole-based chalcones as potent antimicrobial agents. Synthesis and biological evaluation. Bioorg. Med. Chem. 19, 3135–3140 (2011).
Plech, T., Wujec, M., Kosikowska, U., Malm, A., Rajtar, B. & Polz-Dacewicz, M. Synthesis and in vitro activity of 1,2,4-triazole-ciprofloxacin hybrids against drug-susceptible and drug-resistant bacteria. Eur. J. Med. Chem. 60, 128–134 (2013).
Robertson, G. T. et al. In vitro evaluation of CBR-2092, a novel rifamycin-quinolone hybrid antibiotic: studies of the mode of action in Staphylococcus aureus. Antimicrob. Agents Chemother. 52, 2313–2323 (2008).
Vekariya, R. H., Patel, K. D., Rajani, D. P., Rajani, S. D. & Patel, H. D. A one pot, three component synthesis of coumarin hybrid thiosemicarbazone derivatives and their antimicrobial evolution. J. Assoc. Arab Univ. Basic Appl. Sci. 23, 10–15 (2017).
Aggarwal, R., Kumar, S., Kaushik, P., Kaushik, D. & Gupta, G. K. Synthesis and pharmacological evaluation of some novel 2-(5-hydroxy-5-trifluoromethyl-4,5-dihydropyrazol-1-yl)-4-(coumarin-3-yl)thiazole s. Eur. J. Med. Chem. 62, 508–514 (2013).
El Hage, S., Lajoie, B., Feuillolay, C., Roques, C. & Baziard, G. Synthesis, antibacterial and antifungal activities of bifonazole derivatives. Arch. Pharm. 344, 402–410 (2011).
Patel, C. et al. Synthesis and antimicrobial activity of 1,2-benzothiazine derivatives. Molecules 21, 861 (2016).
Mantu, D., Antoci, V., Moldoveanu, C., Zbancioc, G. & Mangalagiu, I. I. Hybrid imidazole (benzimidazole)/pyridine (quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enzyme Inhib. Med. Chem. 31 (sup2), 96–103 (2016).
Kumar, N. P., Nekkanti, S., Sujana Kumari, S., Sharma, P. & Shankaraiah, N. Design and synthesis of 1,2,3-triazolo-phenanthrene hybrids as cytotoxic agents. Bioorg. Med. Chem. Lett. 27, 2369–2376 (2017).
Acknowledgements
We are grateful to the Director, CDRI, Lucknow, India for constant encouragement in drug development program, Dr SP Singh for technical support, SAIF for NMR, IR and Mass spectral data. LRS, ASR, AS and GRP are thankful to CSIR, New Delhi, India for the financial support. SR is thankful to ICMR, New Delhi, India for junior research fellowship. CSIR-CDRI communication number: 302/2015/KVS.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Singh, L., Avula, S., Raj, S. et al. Coumarin–benzimidazole hybrids as a potent antimicrobial agent: synthesis and biological elevation. J Antibiot 70, 954–961 (2017). https://doi.org/10.1038/ja.2017.70
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ja.2017.70
This article is cited by
-
Investigation of recent advancement in the pharmacological potential of C-3 substituted coumarin derivatives against S. aureus
Discover Chemistry (2025)
-
In vitro antimicrobial evaluation and in silico studies of coumarin derivatives tagged with pyrano-pyridine and pyrano-pyrimidine moieties as DNA gyrase inhibitors
Molecular Diversity (2022)
-
Synthesis and evaluation of antimicrobial, antitubercular and anticancer activities of 2-(1-benzoyl-1H-benzo[d]imidazol-2-ylthio)-N-substituted acetamides
Chemistry Central Journal (2018)
-
Coumarin: a novel player in microbial quorum sensing and biofilm formation inhibition
Applied Microbiology and Biotechnology (2018)