Abstract
Cadmium (Cd) has been proposed as a risk factor for age-related macular degeneration (AMD), but the association between Cd exposure and AMD risk in large population studies is unknown. This study evaluated the association of Cd exposure with AMD in a large representative sample of Korean men and women. This was a cross-sectional study of 3865 Korean adults ≥40 years of age who participated in the Korean National Health and Nutrition Examination Survey (KNHANES) during 2008–2011. Cd concentrations in whole blood were measured by graphite-furnace atomic absorption spectrometry. The presence of AMD was determined in digital non-mydriatic fundus photographs. Cd levels were higher in participants with AMD compared with those without AMD (1.3 vs 1.1 μg/l, respectively, P<0.001). In fully adjusted models, the odds ratio for AMD comparing the highest with the lowest Cd quartiles was 1.92 (95% CI=1.08–3.39; P for trend 0.029). In restricted cubic spline models, the association between Cd and AMD was approximately linear, with no evidence of threshold effects. Blood Cd concentrations were independently associated with the prevalence of AMD. If the association is proven causal, population-based preventive strategies to decrease Cd exposure could reduce the population burden of AMD.
Similar content being viewed by others
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of irreversible visual impairment.1 The etiology of AMD is incompletely understood, although several risk factors, including family history, smoking, obesity, sunlight exposure, and nutritional factors have been linked to AMD.2, 3, 4, 5, 6, 7, 8 Smoking, in particular, has been associated with increased AMD risk in a number of studies.2, 3, 4, 5, 6, 7, 8 Because of the high frequency of AMD and the impact of vision loss on reduced quality of life, there is substantial interest in identifying potentially modifying risk factors for AMD that may be amenable to preventive strategies.
Cadmium is a toxic metal9, 10, 11, 12 with widespread population exposure through smoking, diet and ambient air.9, 10, 12 Cadmium (Cd) may promote chronic disease by increasing oxidative stress, through catalysis of reactive oxygen species, by promoting lipid peroxidation and by producing inflammatory cytokines.13, 14 As AMD may be induced by oxidative stress, decreased antioxidant capacity, and inflammation of the choroid and retina, and as Cd accumulation has been identified in retinal cells of patients with smoking-related AMD,15, 16, 17 it has been suggested that Cd exposure may be a risk factor for AMD. Indeed, experimental studies have found that Cd induced toxic effect on human retinal pigment epithelium (RPE) cells indicating possible association between cadmium and AMD.16, 17, 18 Although a small case–control study restricted to smokers found that AMD cases had higher urinary cadmium levels compared with controls without AMD,19 the association of Cd exposure to AMD risk in the general population is unknown. This study thus aims to evaluate association between AMD and environmental cadmium exposure as measured in blood cadmium levels in the Korean National Health and Nutrition Examination Survey (KNHANES), a national representative population survey of Korean adults.
MATERIALS AND METHODS
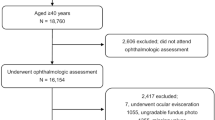
Study Population
KNHANES was a cross-sectional, national representative survey of the non-institutionalized South Korean population conducted by the Korean Ministry of Health and Welfare using a stratified, multistage clustered probability sampling design. Sampling units were defined on the basis of household registries, including geographic area, sex, and age groups. All members of each selected household were asked to participate in the survey, and the participation rate between 2008 and 2011 ranged from 77.8% to 82.8%.
KNHANES includes a health interview, a nutritional survey and a health exam including an ophthalmologic exam, conducted by trained investigators in a specially equipped mobile examination center. Blood samples and full ocular examinations, including blood heavy metal measurements and fundoscopic images, were collected in participants >19 years of age. For this study, we used data from the last two years (2008, 2009) of KNHANES IV (2007–2009) and the first two years (2010, 2011) of KNHANES V (2010–2012) from participants ≥40 years of age who had at least one evaluable fundus photograph, as well as available blood cadmium measurements. The study sample consisted of 1918 men and 1947 women (total 3865 participants). KNHANES was reviewed and approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention, and all participants provided written informed consent. Detailed methods on KNHANES, including survey representativeness and response rate, are available elsewhere.20
Blood Cadmium
Blood samples were collected from each participant after an overnight fast and were processed, immediately refrigerated, and transported to the Central Testing Institute in Seoul, Korea. For heavy metal analyses, 3 ml of whole blood were collected in sodium heparin Vacutainer tubes (Becton, Dickinson & Co, Franklin Lakes, NJ, USA). The analyses were performed at the Neodin Medical Institute, a laboratory certified by the Korean Ministry of Health and Welfare. Cadmium and lead concentrations in whole blood were measured by graphite-furnace atomic absorption spectrometry with Zeeman background correction (Perkin Elmer AAnalyst 600, Turky, Finland). For external quality assurance and control, the Neodin Medical Institute passed the German External Quality Assessment Scheme operated by Friedrich-Alexander University and the Quality Assurance Program operated by the Korea Occupational Safety and Health Agency. For internal quality assurance and control, commercial reference materials were used (Lyphochek Whole Blood Metals Control; Bio-Rad, Hercules, CA, USA). The limit of detection for blood Cd levels was 0.056 μg/l and the coefficients of variation on the basis of four reference samples were within 0.95–4.82%.
Age-Related Macular Degeneration
A digital non-mydriatic fundus camera (TRC-NW6S, Topcon, Tokyo, Japan) and a Nikon D-80 digital camera (Nikon, Tokyo, Japan) were used to obtain digital fundus images under physiological mydriasis. For each participant, a 45° non-mydriatic digital retinal image centered on the fovea (field 2) was taken per eye (two images per person total). If the non-mydriatic photograph was of insufficient quality for grading due to media opacity or a small pupil, a mydriatic fundus photograph was taken after maximal papillary dilation using 1.0% tropicamide and 10% phenylephrine.
Fundus images were graded twice (a preliminary grade and a detailed grade) using the grading protocol of the International Age-related Maculopathy Epidemiological Study Group.21 Participants were defined as having early AMD if they met any one of the following criteria: (1) presence of soft indistinct drusen or reticular drusen; or (2) presence of hard or soft distinct drusen with pigmentary abnormalities (increased pigmentation or hypopigmentation of the retinal pigment epithelium) in the absence of signs of late AMD. Late AMD was defined as the presence of wet AMD or geographic atrophy. Wet AMD was defined as retinal pigment epithelial detachment or serous detachment of the sensory retina, subretinal or sub-RPE hemorrhages, and subretinal fibrous scars. Geographic atrophy was defined as a circular discrete area (175 μm in diameter) of retinal depigmentation with visible choroidal vessels in the absence of signs of wet AMD. When the severity of AMD varied between both eyes of a participant, the most advance grade was assigned to the participant. When only one eye could be definitively assessed, the AMD grade assigned to that participant corresponded to that eye.
Other Variables
Information on age, sex, smoking history, and alcohol intake was collected during the health interview survey. Alcohol consumption was indicated as “yes” for participants who consumed at least one glass of alcohol every month over the last year. Smoking status was divided into never, former, and current. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mm Hg, and/or current use of blood pressure-lowering medications. Diabetes was defined as a fasting plasma glucose ≥126 mg/dl, and/or current use of oral hypoglycemic agents or insulin.
Statistical Methods
Survey weights were calculated to account for survey year, stage of selection, and non-response. Statistical analysis used the survey commands of Stata (release 12.1; StataCorp LP, College Station, TX, USA) to account for survey weights and for the complex sampling design. The distribution of blood Cd was right skewed and log-transformed for the analysis. Participants were divided into quartiles of cadmium concentration on the basis of the weighted population distribution. We used multivariable logistic regression to calculate odds ratios (ORs) for AMD comparing each quartile of blood cadmium with the lowest quartile. We used three models with progressive degrees of adjustment. Statistical models were initially adjusted for age (continuous) and sex (male or female). Logistic regression models were further adjusted for smoking status (never, former, or current) and alcohol consumption (yes or no). Tests for linear trend were computed by including a variable with the median values of cadmium in each quartile as a continuous variable in the logistic regression models.
To further explore the shape of the dose–response relationship between cadmium and AMD, we used restricted cubic splines with knots at the 10th, 50th, and 90th percentiles of the weighted blood Cd distribution. We also evaluated the interactions between Cd (modeled as a continuous log-transformed variable) and sex, age groups, alcohol consumption, smoking status, and diabetes status. Two-sided P-values <0.05 were considered statistically significant.
RESULTS
The mean age of study participants was 52.6 years and the overall prevalence of AMD was 5.4% (95% CI=4.6%–6.3%; Table 1). Participants with AMD were more likely to be older (P<0.001) and less likely to be alcohol drinkers (P=0.006) compared with participants without AMD. The geometric mean Cd concentration in the study population was 1.2 μg/l. Women had higher geometric mean Cd concentrations than men (1.3 vs 1.0 μg/l, respectively), and current smokers had higher geometric mean Cd concentrations compared with former and never smokers (1.2, 0.9, and 1.2 μg/l, respectively; Table 2). Cd levels were positively associated with age, female sex, blood lead, and hypertension, and negatively associated with alcohol consumption.
Cd levels were higher in participants with AMD compared with those without AMD (geometric means 1.3 vs 1.1 μg/l, P<0.001). In fully adjusted logistic regression models, the odds ratio for AMD comparing the highest (≥1.63 μg/l) with the lowest (<0.84 μg/l) Cd quartile was 1.92 (95% CI=1.08–3.39; P for trend 0.029; Table 3). Additional analyses using urine cotinine to classify current smokers and to identify participants exposed to second hand smoke were virtually the same as those based on smoking categorization using self-report (not shown). In fully adjusted restricted cubic spline models, the OR for AMD increased progressively with increasing cadmium concentrations up to 5 μg/l (Figure 1). The dose–response relationship between Cd and the prevalence of AMD was approximately linear with no clear threshold effects. When using log-Cd as a continuous variable in fully adjusted models, the adjusted ORs for AMD comparing the 90th (2.17 μg/l) with the 10th (0.61 μg/l) percentile of the Cd distribution was 1.65 (95% CI=1.04–2.63; Figure 2). Further adjustment for blood lead concentrations produced similar results. Subgroup analyses showed that the association between Cd and AMD was stronger in participants <60 years of age compared with those ≥60 years of age, and although the interaction test was not statistically significant, the association appeared to be stronger in participants with diabetes compared with those without diabetes (Figure 2). The association was consistent across other clinically relevant subgroups.
Odds ratios (solid curve) and 95% confidence intervals (dotted curves) for age-related macular degeneration by blood cadmium concentrations. Curves represent adjusted odds ratios on the basis of restricted cubic splines for cadmium levels with knots at the 10th, 50th, and 90th percentiles of the weighted sample distributions. The reference cadmium concentration (diamond) was set at the 10th percentile of the blood cadmium distribution (0.61 μg/l). Results were obtained from multivariable logistic regression models adjusted for age, sex, smoking status, and alcohol drinking.
Odds ratios (95% confidence intervals) for age-related macular degeneration comparing the 90th (2.17 μg/l) with the 10th percentile (0.61 μg/l) of the blood cadmium distribution. Cadmium was modeled as log-linear term. Multivariable logistic regression models were adjusted for age, sex, smoking status, and alcohol drinking. The size of the square is proportional to the sample size in each stratum. P-values correspond to tests for interaction between cadmium splines and selected participant characteristics.
DISCUSSION
In this large, representative sample of Korean adults, increased blood Cd levels were independently associated with the prevalence of AMD. Participants in the highest quartile of blood Cd were almost two times more likely to have AMD compared with those in the lowest quartile. The association was approximately linear, with no evidence of threshold effects, and remained present across subgroups defined by sex, race, smoking, drinking status, and diabetes mellitus. Our findings add to the concern that Cd exposure may contribute to the development of AMD in the general population.
Cadmium is a persistent environmental pollutant with increasing levels in air, water, and soil during the 20th century.22 The main sources of Cd exposure in the general population are cigarette smoke, certain foods including shellfish, organ meats, grains, and root vegetables, and industrial releases from smelting and fuel combustion. Once absorbed by the human body, Cd accumulates in the kidneys and in other organs with a biological half-life well beyond 10 years.23 Blood Cd reflects current exogenous exposure, as well as chronic endogenous exposure from accumulated body burdens. Cd exposure has been proposed as a risk factor for kidney disease, hypertension, diabetes, cardiovascular diseases, cancer, blood pressure, and reproductive outcomes.24
Erie et al.15 found that human ocular tissues, particularly retinal pigment epithelium and choroid tissues, accumulated Cd at levels higher than the blood or ocular fluids, and that Cd concentrations were four times higher in retinal tissue of smokers compared with non-smokers. In addition, they found that urine Cd levels, reflecting total body burden of Cd, were higher in smokers with AMD compared with smokers without AMD.19 Recently, experimental reports have confirmed cadmium accumulation in the human retina,16, 17, 25 and the role of Cd exposure in the development and progression of AMD has been a source of increasing concern especially among smokers.
A small case–control study19 reported that urine Cd levels were associated with an increased risk of AMD in smokers, but not in non-smokers. In the present study, with a much larger sample size, the association between blood Cd and AMD was similar in never, former, and current smokers, suggesting that Cd is related to AMD irrespective of the source of exposure.
The geometric mean blood Cd concentration in our study population was 1.2 μg/l, substantially higher than current concentrations in western countries. In a general population survey of over 4000 adults in Germany, the mean blood cadmium concentration was 0.44 μg/l,26 whereas the mean blood Cd in the US population reported in the 4th National Report on Human Exposure to Environmental Chemicals was 0.41 μg/l, with a 95th percentile of 1.3 μg/l.27 In addition to a high frequency of smoking in men, the higher blood Cd levels in the Korean population may be related to the intake of foods with high Cd levels, including rice.28, 29 Moon et al.30 reported that the mean dietary Cd intake in Korea was 21.2 μg/day, a much larger quantity than many other countries, with Cd intake from boiled rice accounting for 23% of total Cd intake. Reducing Cd exposure in the Korean population will thus have to be based on a combination of reducing the prevalence of smoking and reducing dietary intake by avoiding foods contaminated with Cd.
The precise mechanisms underlying the association between Cd and AMD have not been identified. The toxic effects of Cd may be mediated by increased levels of reactive oxygen species31 and other oxidative damage through the binding of cadmium to sulfhydryl groups, the depletion of glutathione, and the production of inflammatory cytokines.32, 33 Indeed, there is an increasing evidence on the role of oxidative stress in the pathogenesis of AMD.34, 35, 36 Although our study cannot identify the mechanisms underlying the association between Cd and AMD, our data suggest that Cd is an independent risk factor for AMD and support additional research to establish the pathways involved in Cd-related AMD.
Several limitations need to be considered in the interpretation of our results. We used a cross-sectional design, which limits the assessment of temporal relationships, as well as causal inferences. We also used a single blood Cd measurement, which may be subject to random misclassification of long-term Cd exposure and consequently underestimate the study associations. In addition, KNHANES did not include an occupation/job code to identify jobs that may be affected by occupational Cd exposure such as smelting, iron/steel production, and mining. Finally, our study used a national representative sample of Korean men and women, but our findings may not be generalizable to the residents in other countries or to subjects of other race/ethnicities. In spite of these limitations, the representative nature of the sample, the strict quality control procedures of the KNHANES field and laboratory methods, the performance of a detailed ophthalmologic examination, the availability of data on a number of potential confounders, and the large sample size are important strengths of our study that add plausibility to the findings.
In conclusion, Cd exposure was associated with an increased risk of AMD in a national representative sample of Korean men and women. AMD is a major cause of vision loss, reduced quality of life, disability, and medical costs. If the association between Cd and AMD is established as causal, population-based preventive strategies to decrease Cd exposure could reduce the population burden of AMD.
References
Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004; 122: 564–572.
Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001; 119: 1417–1436.
Mares-Perlman JA, Brady WE, Klein R, Klein BE, Bowen P, Stacewicz-Sapuntzakis M et al. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol 1995; 113: 1518–1523.
Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994; 272: 1413–1420.
Seddon JM, Cote J, Davis N, Rosner B . Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol 2003; 121: 785–792.
Seddon JM, Cote J, Rosner B . Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003; 121: 1728–1737.
Seddon JM, Willett WC, Speizer FE, Hankinson SE . A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 1996; 276: 1141–1146.
Tomany SC, Wang JJ, Van Leeuwen R, Klein R, Mitchell P, Vingerling JR et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology 2004; 111: 1280–1287.
IARC Working group on the evaluation of carcinogenic risks to humans. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. IARC Monogr Eval Carcinog Risks Hum 1993; 58: 1–415.
Agency for Toxic Substances and Disease Registry (ATSDR), 2012. Toxicological profile for Cadmium. Available at http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15/ Accessed: 17 November 2013.
U.S. Geological survey Mineral Commodity Summaries 2010. United States Government Printing Office: Washington, USA. 2010.
Nordberg GF, Nogawa K, Nordberg M, Friberg L . Cadmium. Nordberg GF, Fowler BF, Nordberg M, Friberg L. Handbook on the Toxicology of Metals. Elsevier: Amsterdam, The Netherlands. 2007. pp 445–486.
Jin T, Lu J, Nordberg M . Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology 1998; 19: 529–535.
Valko M, Morris H, Cronin MT . Metals, toxicity and oxidative stress. Curr Med Chem 2005; 12: 1161–1208.
Erie JC, Butz JA, Good JA, Erie EA, Burritt MF, Cameron JD . Heavy metal concentrations in human eyes. Am J Ophthalmol 2005; 139: 888–893.
Wills NK, Kalariya N, Sadagopa Ramanujam VM, Lewis JR, Haji Abdollahi S, Husain A et al. Human retinal cadmium accumulation as a factor in the etiology of age-related macular degeneration. Exp Eye Res 2009; 89: 79–87.
Wills NK, Ramanujam VM, Chang J, Kalariya N, Lewis JR, Weng TX et al. Cadmium accumulation in the human retina: effects of age, gender, and cellular toxicity. Exp Eye Res 2008; 86: 41–51.
Kalariya NM, Wills NK, Ramana KV, Srivastava SK, van Kuijk FJ . Cadmium-induced apoptotic death of human retinal pigment epithelial cells is mediated by MAPK pathway. Exp Eye Res 2009; 89: 494–502.
Erie JC, Good JA, Butz JA, Hodge DO, Pulido JS . Urinary cadmium and age-related macular degeneration. Am J Ophthalmol 2007; 144: 414–418.
Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014; 43: 69–77.
Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 1995; 39: 367–374.
Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M . Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand J Work Environ Health 1998; 24 (Suppl 1): 1–51.
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ et al. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 2003; 137: 65–83.
Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals 2010; 23: 769–782.
Wills NK, Ramanujam VM, Kalariya N, Lewis JR, van Kuijk FJ . Copper and zinc distribution in the human retina: relationship to cadmium accumulation, age, and gender. Exp Eye Res 2008; 87: 80–88.
Becker K, Schulz C, Kaus S, Seiwert M, Seifert B . German Environmental Survey 1998 (GerES III): environmental pollutants in the urine of the German population. Int J Hyg Environ Health 2003; 206: 15–24.
Small RG . Stabilization of eyelid height after aponeurotic ptosis repair. Ophthalmology 1999; 106: 2043–2044.
Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G . Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003-2004. Environ Health Perspect 2010; 118: 1735–1742.
Moon CS, Paik JM, Choi CS, Kim DH, Ikeda M . Lead and cadmium levels in daily foods, blood and urine in children and their mothers in Korea. Int Arch Occup Environ Health 2003; 76: 282–288.
Moon CS, Zhang ZW, Shimbo S, Watanabe T, Moon DH, Lee CU et al. Dietary intake of cadmium and lead among the general population in Korea. Environ Res 1995; 71: 46–54.
Bertin G, Averbeck D . Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006; 88: 1549–1559.
Stohs SJ, Bagchi D . Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 1995; 18: 321–336.
Yiin SJ, Chern CL, Sheu JY, Tseng WC, Lin TH . Cadmium-induced renal lipid peroxidation in rats and protection by selenium. J Toxicol Environ Health A 1999; 57: 403–413.
Beatty S, Koh H, Phil M, Henson D, Boulton M . The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000; 45: 115–134.
Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP . Oxidative damage and protection of the RPE. Prog Retin Eye Res 2000; 19: 205–221.
Winkler BS, Boulton ME, Gottsch JD, Sternberg P . Oxidative damage and age-related macular degeneration. Mol Vis 1999; 5: 32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, M., Zhao, D., Cho, J. et al. Cadmium exposure and age-related macular degeneration. J Expo Sci Environ Epidemiol 26, 214–218 (2016). https://doi.org/10.1038/jes.2014.75
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jes.2014.75
Keywords
This article is cited by
-
Estimation of health risks associated with dietary cadmium exposure
Archives of Toxicology (2023)