Abstract
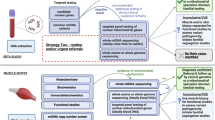
Sensorineural hearing loss (HL) is one of the most frequent clinical features in patients with mitochondrial diseases caused by mitochondrial DNA (mtDNA) mutations, and hearing is impaired in over half of all cases with mitochondrial disorders. This study analyzed 373 patients with suspected hereditary HL using an extensive and rapid suspension-array screening system for 29 major mtDNA mutations, including the m.1555A>G homoplasmic mutation in the MT-RNR1 gene, which causes non-syndromic sensorineural HL and aminoglycoside-induced HL, and the m.3243A>G heteroplasmic mutation in the MT-TL1 gene. This method is rapid and suitable for large-scale screening because universal 96-well plates are available for use, and because an analysis of each plate can be completed within 1 h. This system detected five different mtDNA mutations in 24 of the 373 (6.4%) patients. The m.1555A>G and m.3243A>G mutations were detected in 11 (2.9%) and 9 (2.7%) patients, respectively. In addition, three mutations, that is, m.8348A>G in the MT-TK gene, m.11778G>A in the MT-ND4 gene and 15498G>A in the MT-CYB gene were detected in one patient for each. This screening system is useful for the genetic diagnosis and epidemiological study of both syndromic and non-syndromic HL.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Morton, C. C. & Nance, W. E. Newborn hearing screening—a silent revolution. N. Engl. J. Med. 354, 2151–2164 (2006).
Chinnery, P. F., Elliott, C., Green, G. R., Rees, A., Coulthard, A., Turnbull, D. M. et al. The spectrum of hearing loss due to mitochondrial DNA defects. Brain 123, 82–92 (2000).
Hutchin, T. P. & Cortopassi, G. A. Mitochondrial defects and hearing loss. Cell Mol. Life Sci. 57, 1927–1937 (2000).
Taylor, R. W. & Turnbull, D. M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6, 389–402 (2005).
Van Camp, G. & Smith, R. J. Maternally inherited hearing impairment. Clin. Genet. 57, 409–414 (2000).
Xing, G., Chen, Z. & Cao, X. Mitochondrial rRNA and tRNA and hearing function. Cell Res. 17, 227–239 (2007).
Casano, R. A., Bykhovskaya, Y., Johnson, D. F., Hamon, M., Torricelli, F., Bigozzi, M. et al. Hearing loss due to the mitochondrial A1555G mutation in Italian families. Am. J. Med. Genet. 79, 388–391 (1998).
Fischel-Ghodsian, N., Prezant, T. R., Bu, X. & Oztas, S. Mitochondrial ribosomal RNA gene mutation in a patient with sporadic aminoglycoside ototoxicity. Am. J. Otolaryngol. 14, 399–403 (1993).
Prezant, T. R., Agapian, J. V., Bohlman, M. C., Bu, X., Oztas, S., Qiu, W. Q. et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat. Genet. 4, 289–294 (1993).
Bravo, O., Ballana, E. & Estivill, X. Cochlear alterations in deaf and unaffected subjects carrying the deafness-associated A1555G mutation in the mitochondrial 12S rRNA gene. Biochem. Biophys. Res. Commun. 344, 511–516 (2006).
Estivill, X., Govea, N., Barcelo, E., Badenas, C., Romero, E., Moral, L. et al. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment of aminoglycosides. Am. J. Hum. Genet. 62, 27–35 (1998).
Noguchi, Y., Yashima, T., Ito, T., Sumi, T., Tsuzuku, T. & Kitamura, K. Audiovestibular findings in patients with mitochondrial A1555G mutation. Laryngoscope 114, 344–348 (2004).
Schapira, A. H. Mitochondrial disease. Lancet 368, 70–82 (2006).
van den Ouweland, J. M., Lemkes, H. H., Ruitenbeek, W., Sandkuijl, L. A., de Vijlder, M. F., Struyvenberg, P. A. et al. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat. Genet. 1, 368–371 (1992).
Tamagawa, Y., Kitamura, K., Hagiwara, H., Ishida, T., Nishizawa, M., Saito, T. et al. Audiologic findings in patients with a point mutation at nucleotide 3243 of mitochondrial DNA. Ann. Otol. Rhinol. Laryngol. 106, 338–342 (1997).
Fuku, N., Park, K. S., Yamada, Y., Nishigaki, Y., Cho, Y. M., Matsuo, H. et al. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am. J. Hum. Genet. 80, 407–415 (2007).
Nishigaki, Y., Yamada, Y., Fuku, N., Matsuo, H., Segawa, T., Watanabe, S. et al. Mitochondrial haplogroup A is a genetic risk factor for atherothrombotic cerebral infarction in Japanese females. Mitochondrion 7, 72–79 (2007).
Nishigaki, Y., Yamada, Y., Fuku, N., Matsuo, H., Segawa, T., Watanabe, S. et al. Mitochondrial haplogroup N9b is protective against myocardial infarction in Japanese males. Hum. Genet. 120, 827–836 (2007).
Tanaka, M., Fuku, N., Nishigaki, Y., Matsuo, H., Segawa, T., Watanabe, S. et al. Women with mitochondrial haplogroup N9a are protected against metabolic syndrome. Diabetes 56, 518–521 (2007).
Nishigaki, Y., Ueno, H., Coku, J., Koga, Y., Fujii, T., Sahashi, K. et al. Extensive screening system using suspension array technology to detect mitochondrial DNA point mutations. Mitochondrion, in press (2009).
Tiranti, V., D'Agruma, L., Pareyson, D., Mora, M., Carrara, F., Zelante, L. et al. A novel mutation in the mitochondrial tRNA(Val) gene associated with a complex neurological presentation. Ann. Neurol. 43, 98–101 (1998).
Andreu, A. L., Hanna, M. G., Reichmann, H., Bruno, C., Penn, A. S., Tanji, K. et al. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N. Engl. J. Med. 341, 1037–1044 (1999).
Dunbar, S. A. Applications of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin. Chim. Acta. 363, 71–82 (2006).
Ye, F., Li, M. S., Taylor, J. D., Nguyen, Q., Colton, H. M., Casey, W. M. et al. Fluorescent microsphere-based readout technology for multiplexed human single nucleotide polymorphism analysis and bacterial identification. Hum. Mutat. 17, 305–316 (2001).
Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H., Coulson, A. R., Drouin, J. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981).
Andrews, R. M., Kubacka, I., Chinnery, P. F., Lightowlers, R. N., Turnbull, D. M. & Howell, N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 23, 147 (1999).
Nishigaki, Y., Marti, R., Copeland, W. C. & Hirano, M. Site-specific somatic mitochondrial DNA point mutations in patients with thymidine phosphorylase deficiency. J. Clin. Invest. 111, 1913–1921 (2003).
Ueno, H., Nishigaki, Y., Kong, Q. P., Fuku, N., Kojima, S., Iwata, N. et al. Analysis of mitochondrial DNA variants in Japanese patients with schizophrenia. Mitochondrion 9, 385–393 (2009).
Vandebona, H., Mitchell, P., Manwaring, N., Griffiths, K., Gopinath, B., Wang, J. J. et al. Prevalence of mitochondrial 1555A → G mutation in adults of European descent. N. Engl. J. Med. 360, 642–644 (2009).
Usami, S., Abe, S., Akita, J., Namba, A., Shinkawa, H., Ishii, M. et al. Prevalence of mitochondrial gene mutations among hearing impaired patients. J. Med. Genet. 37, 38–40 (2000).
Goto, Y., Nonaka, I. & Horai, S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348, 651–653 (1990).
Pavlakis, S. G., Phillips, P. C., DiMauro, S., De Vivo, D. C. & Rowland, L. P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann. Neurol. 16, 481–488 (1984).
Sotiriou, E., Coku, J., Tanji, K., Huang, H. B., Hirano, M. & DiMauro, S. The m.3244G>A mutation in mtDNA is another cause of progressive external ophthalmoplegia. Neuromuscul. Disord. 19, 297–299 (2009).
Chinnery, P. F., Johnson, M. A., Wardell, T. M., Singh-Kler, R., Hayes, C., Brown, D. T. et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann. Neurol. 48, 188–193 (2000).
McFarland, R., Taylor, R. W. & Turnbull, D. M. The neurology of mitochondrial DNA disease. Lancet Neurol. 1, 343–351 (2002).
Schaefer, A. M., Taylor, R. W., Turnbull, D. M. & Chinnery, P. F. The epidemiology of mitochondrial disorders—past, present and future. Biochim. Biophys. Acta. 1659, 115–120 (2004).
Manwaring, N., Jones, M. M., Wang, J. J., Rochtchina, E., Howard, C., Mitchell, P. et al. Population prevalence of the MELAS A3243G mutation. Mitochondrion 7, 230–233 (2007).
Majamaa, K., Moilanen, J. S., Uimonen, S., Remes, A. M., Salmela, P. I., Karppa, M. et al. Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am. J. Hum. Genet. 63, 447–454 (1998).
Oshima, T., Ueda, N., Ikeda, K., Abe, K. & Takasaka, T. Hearing loss with a mitochondrial gene mutation is highly prevalent in Japan. Laryngoscope 109, 334–338 (1999).
Terasaki, F., Tanaka, M., Kawamura, K., Kanzaki, Y., Okabe, M., Hayashi, T. et al. A case of cardiomyopathy showing progression from the hypertrophic to the dilated form: association of Mt8348A → G mutation in the mitochondrial tRNA(Lys) gene with severe ultrastructural alterations of mitochondria in cardiomyocytes. Jpn. Circ. J. 65, 691–694 (2001).
Achilli, A., Rengo, C., Magri, C., Battaglia, V., Olivieri, A., Scozzari, R. et al. The molecular dissection of mtDNA haplogroup H confirms that the Franco-Cantabrian glacial refuge was a major source for the European gene pool. Am. J. Hum. Genet. 75, 910–918 (2004).
Sissler, M., Helm, M., Frugier, M., Giege, R. & Florentz, C. Aminoacylation properties of pathology-related human mitochondrial tRNA(Lys) variants. RNA 10, 841–853 (2004).
Man, P. Y., Griffiths, P. G., Brown, D. T., Howell, N., Turnbull, D. M. & Chinnery, P. F. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet. 72, 333–339 (2003).
Wallace, D. C., Singh, G., Lott, M. T., Hodge, J. A., Schurr, T. G., Lezza, A. M. et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science 242, 1427–1430 (1988).
DiMauro, S. & Schon, E. A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 348, 2656–2668 (2003).
Yu-Wai-Man, P., Elliott, C., Griffiths, P. G., Johnson, I. J. & Chinnery, P. F. Investigation of auditory dysfunction in Leber hereditary optic neuropathy. Acta. Ophthalmol. 86, 630–633 (2008).
Ceranic, B. & Luxon, L. M. Progressive auditory neuropathy in patients with Leber's hereditary optic neuropathy. J. Neurol. Neurosurg. Psychiatry 75, 626–630 (2004).
Andreu, A. L., Checcarelli, N., Iwata, S., Shanske, S. & DiMauro, S. A missense mutation in the mitochondrial cytochrome b gene in a revisited case with histiocytoid cardiomyopathy. Pediatr. Res. 48, 311–314 (2000).
Finsterer, J. Histiocytoid cardiomyopathy: a mitochondrial disorder. Clin. Cardiol. 31, 225–227 (2008).
Konings, A., Van Camp, G., Goethals, A., Van Eyken, E., Vandevelde, A., Ben Azza, J. et al. Mutation analysis of mitochondrial DNA 12SrRNA and tRNASer(UCN) genes in non-syndromic hearing loss patients. Mitochondrion 8, 377–382 (2008).
Meierhofer, D., Mayr, J. A., Ebner, S., Sperl, W. & Kofler, B. Rapid screening of the entire mitochondrial DNA for low-level heteroplasmic mutations. Mitochondrion 5, 282–296 (2005).
Bannwarth, S., Procaccio, V., Rouzier, C., Fragaki, K., Poole, J., Chabrol, B. et al. Rapid identification of mitochondrial DNA (mtDNA) mutations in neuromuscular disorders by using surveyor strategy. Mitochondrion 8, 136–145 (2008).
Mashima, Y., Nagano, M., Funayama, T., Zhang, Q., Egashira, T., Kudho, J. et al. Rapid quantification of the heteroplasmy of mutant mitochondrial DNAs in Leber's hereditary optic neuropathy using the Invader technology. Clin. Biochem. 37, 268–276 (2004).
Elliott, H. R., Samuels, D. C., Eden, J. A., Relton, C. L. & Chinnery, P. F. Pathogenic mitochondrial DNA mutations are common in the general population. Am. J. Hum. Genet. 83, 254–260 (2008).
White, H. E., Durston, V. J., Seller, A., Fratter, C., Harvey, J. F. & Cross, N. C. Accurate detection and quantitation of heteroplasmic mitochondrial point mutations by pyrosequencing. Genet. Test. 9, 190–199 (2005).
Leveque, M., Marlin, S., Jonard, L., Procaccio, V., Reynier, P., Amati-Bonneau, P. et al. Whole mitochondrial genome screening in maternally inherited non-syndromic hearing impairment using a microarray resequencing mitochondrial DNA chip. Eur. J. Hum. Genet. 15, 1145–1155 (2007).
Maitra, A., Cohen, Y., Gillespie, S. E., Mambo, E., Fukushima, N., Hoque, M. O. et al. The Human MitoChip: a high-throughput sequencing microarray for mitochondrial mutation detection. Genome Res. 14, 812–819 (2004).
Acknowledgements
We thank Y Abe for helpful discussions and excellent technical support. This study was supported in part by a grants from the program Grants-in-Aid for Scientific Research (C) (18590317 to Y Nishigaki, and 21590411 to HH); by grant 20B-13 from the program Research Grants for Nervous and Mental Disorders of the Ministry of Health, Labour, and Welfare (to MT); Research on Measures for Intractale Diseases) from the Ministry of Health, Labour and Welfare of Japan (to KK); 21st Century COE Program Brain Integration and Its Disorder (to KK); and by grants for scientific research from the Takeda Science Foundation (to Y Nishigaki and MT), and Sankyo Foundation of Life Science (to Y Nishigaki).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website (http://www.nature.com/jhg)
Supplementary information
Rights and permissions
About this article
Cite this article
Kato, T., Nishigaki, Y., Noguchi, Y. et al. Extensive and rapid screening for major mitochondrial DNA point mutations in patients with hereditary hearing loss. J Hum Genet 55, 147–154 (2010). https://doi.org/10.1038/jhg.2009.143
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2009.143
Keywords
This article is cited by
-
Extended screening for major mitochondrial DNA point mutations in patients with hereditary hearing loss
Journal of Human Genetics (2012)