Abstract
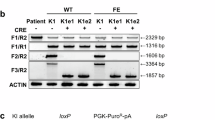
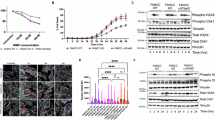
Human artificial chromosome (HAC) has several advantages as a gene therapy vector, including stable episomal maintenance that avoids insertional mutations and the ability to carry large gene inserts. To examine the copy number effect on the gene expression levels and its stability for a long-term culture for a future application in gene therapy, we constructed a HAC vector carrying the human factor VIII (FVIII) complementary DNA, FVIII-HAC in Chinese hamster ovary (CHO) cells. One and more copies of FVIII gene on the HAC were expressed in the copy-number-dependent manner in the CHO cells. The HAC with 16 copies of FVIII, FVIII (16)-HAC, was transferred from CHO hybrids into a human immortalized mesenchymal stem cell using microcell-mediated chromosome transfer. The expression levels of HAC-derived FVIII transgene products were compared with transfected FVIII plasmids. The former showed expression levels consistent with those of the original clones, even after 50 population doublings, whereas the latter showed a remarkable decrease in expression despite unvarying DNA content, indicating that the gene on the HAC is resistant to gene silencing. These results suggest that the HAC-mediated therapeutic gene-expression system may be a powerful tool for stable expression of transgenes, and possibly for industrial production of gene products.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Murphy, S. L. & High, K. A. Gene therapy for haemophilia. Br. J. Haematol. 140, 479–487 (2008).
Basu, J. & Willard, H. F. Artificial and engineered chromosomes: non-integrating vectors for gene therapy. Trends Mol. Med. 11, 251–258 (2005).
Oshimura, M. & Katoh, M. Transfer of human artificial chromosome vectors into stem cells. Reprod. Biomed. Online 16, 57–69 (2008).
Katoh, M., Ayabe, F., Norikane, S., Okada, T., Masumoto, H., Horike, S. et al. Construction of a novel human artificial chromosome vector for gene delivery. Biochem. Biophys. Res. Commun. 321, 280–290 (2004).
Hoshiya, H., Kazuki, Y., Abe, S., Takiguchi, M., Kajitani, N., Watanabe, Y. et al. A highly stable and nonintegrated human artificial chromosome (HAC) containing the 2.4 Mb entire human dystrophin gene. Mole. Ther. 17, 309–317 (2009).
Kazuki, Y., Hiratsuka, M., Takiguchi, M., Osaki, M., Kajitani, N., Hoshiya, H. et al. Complete genetic correction of iPS cells from duchenne muscular dystrophy. Mole. Ther. 18, 386–393 (2010).
Kazuki, Y., Hoshiya, H., Kai, Y., Abe, S., Takiguchi, M., Osaki, M. et al. Correction of a genetic defect in multipotent germline stem cells using a human artificial chromosome. Gene Ther. 15, 617–624 (2008).
Ren, X. Y., Tahimic, C. G. T., Katoh, M., Kurimasa, A., Inoue, T. & Oshimura, M. Human artificial chromosome vectors meet stem cells - new prospects for gene delivery. Stem Cell Rev. 2, 43–50 (2006).
Kazuki, Y., Hoshiya, H., Takiguchi, M., Abe, S., Iida, Y., Osaki, M. et al. Refined human artificial chromosome vectors for gene therapy and animal transgenesis. Gene Ther. 18, 384–393 (2011).
Okamoto, T., Aoyama, T., Nakayama, T., Nakamata, T., Hosaka, T., Nishijo, K. et al. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 295, 354–361 (2002).
Chung, J. H., Bell, A. C. & Felsenfeld, G. Characterization of the chicken beta-globin insulator. Proc. Natl Acad. Sci. U S A 94, 575–580 (1997).
Recillas-Targa, F., Pikaart, M J., Burgess-Beusse, B., Bell, A C., Litt, M D., West, A G. et al. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc. Natl Acad. Sci. U S A 99, 6883–6888 (2002).
Niwa, H., Yamamura, K. & Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–199 (1991).
Ogata, K., Mimuro, J., Kikuchi, J., Tabata, T., Ueda, Y., Naito, M. et al. Expression of human coagulation factor VIII in adipocytes transduced with the simian immunodeficiency virus agmTYO1-based vector for hemophilia A gene therapy. Gene Ther. 11, 253–259 (2004).
Bi, L., Lawler, A. M., Antonarakis, S. E., High, K. A., Gearhart, J. D. & Kazazian, H. H. Targeted disruption of the mouse factor-viii gene produces a model of hemophilia-A. Nat. Genet. 10, 119–121 (1995).
Roth, D. A., Tawa, N. E., O’Brien, J. M., Treco, D. A., Selden, R. F. & Factor, V. T. T. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N. Engl. J. Med. 344, 1735–1742 (2001).
Wilson, C., Bellen, H. J. & Gehring, W. J. Position effects on eukaryotic gene-expression. Ann. Rev. Cell Biol. 6, 679–714 (1990).
Ellis, J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 16, 1241–1246 (2005).
Hong, H., Takahashi, K., Ichisaka, T., Aoi, T., Kanagawa, O., Nakagawa, M. et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 460, 1132–U1195 (2009).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Okita, K., Nakagawa, M., Hong, H. J., Ichisaka, T. & Yamanaka, S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 322, 949–953 (2008).
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Wurm, F M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 22, 1393–1398 (2004).
Herlitschka, S. E., Schlokat, U., Falkner, F. G. & Dorner, F. High expression of a B-domain deleted factor VIII gene in a human hepatic cell line. J. Biotechnol. 61, 165–173 (1998).
Campos-da-Paz, M., Costa, C. S., Quilici, L. S., de Carmo Simões, I., Kyaw, C. M., Maranhão, A. Q. et al. Production of recombinant human factor VIII in different cell lines and the effect of human XBP1 co-expression. Mol. Biotechnol. 39, 155–158 (2008).
Acknowledgements
We wish to thank Drs M Okabe (Osaka University) for providing pCX-EGFP; G Felsenfeld, (National Institutes of Health) for providing pJC5-4. This study was supported in part by a Health and Labor Science Research Grant for Research on HIV/AIDS and Research on Intractable Diseases from the Japanese Ministry of Health, Labour and Welfare, a Grant-in-Aid for Research Activity Start-up, and a Regional Innovation Cluster Program grant (accelerative support) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. All animal studies were approved by the Institutional Animal Care and Use Committee of Tottori University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Kurosaki, H., Hiratsuka, M., Imaoka, N. et al. Integration-free and stable expression of FVIII using a human artificial chromosome. J Hum Genet 56, 727–733 (2011). https://doi.org/10.1038/jhg.2011.88
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2011.88
Keywords
This article is cited by
-
An efficient protein production system via gene amplification on a human artificial chromosome and the chromosome transfer to CHO cells
Scientific Reports (2019)
-
Combinations of chromosome transfer and genome editing for the development of cell/animal models of human disease and humanized animal models
Journal of Human Genetics (2018)
-
Moving toward a higher efficiency of microcell-mediated chromosome transfer
Molecular Therapy - Methods & Clinical Development (2016)
-
Retargeting of microcell fusion towards recipient cell-oriented transfer of human artificial chromosome
BMC Biotechnology (2015)
-
A pathway from chromosome transfer to engineering resulting in human and mouse artificial chromosomes for a variety of applications to bio-medical challenges
Chromosome Research (2015)