Abstract
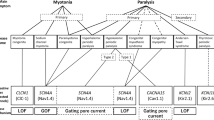
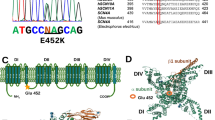
Myotonia congenita is a genetic disease characterized by impaired muscle relaxation after forceful contraction (myotonia) and caused by mutations in the chloride channel voltage-sensitive 1 (CLCN1) gene, encoding the voltage-gated chloride channel of skeletal muscle (ClC-1). In a large cohort of clinically diagnosed unrelated probands, we identified 75 different CLCN1 mutations in 106 individuals, among which 29 were novel mutations and 46 had already been reported. Despite the newly described mutations being scattered throughout the gene, in our patients, mutations were mostly found in exons 4 and 5. Most of the novel mutations located in the region comprising the intramembrane helices are involved in the ion-conducting pathway and predicted to affect channel function. We report for the first time that two mutations, inherited on the same allele as a heterozygous trait, abrogate disease expression, although when inherited singularly they were pathogenic. Such a mode of inheritance might explain the incomplete penetrance reported for autosomal dominant mutations in particular families.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Becker, P. Myotonia Congenita and Syndromes Associated with Myotonia, (Thieme: Stuttgart, 1977).
Thomsen, J. Tonische Krämpfe in willkürlich beweglichen Muskeln in Folge von ererbter psychischer disposition. Arch. Psychiatr. Nervenkr. 6, 702–718 (1876).
Lossin, C. & George, A. L. Jr. Myotonia congenita. Adv. Genet. 63, 25–55 (2008).
Lehmann-Horn, F., Jurkat-Rott, K. & Rüdel, R. Ulm Muscle Centre: diagnostics and therapy of muscle channelopathies—guidelines of the Ulm Muscle Centre. Acta Myol. 27, 98–113 (2008).
Lorenz, C., Meyer-Kleine, C., Steinmeyer, K., Koch, M. C. & Jentsch, T. J. Genomic organization of the human muscle chloride channel ClC-1 and analysis of novel mutations leading to Becker-type myotonia. Hum. Mol. Genet. 3, 941–946 (1994).
Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T. & MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415, 287–294 (2002).
Estévez, R., Pusch, M., Ferrer-Costa, C., Orozco, M. & Jentsch, T. J. Functional and structural conservation of CBS domains from CLC chloride channels. J. Physiol. 557, 363–378 (2004).
Kubisch, C., Schmidt-Rose, T., Fontaine, B., Bretag, A. H. & Jentsch, T. J. ClC-1 chloride channel mutations in myotonia congenita: variable penetrance of mutations shifting the voltage dependence. Hum. Mol. Genet. 7, 1753–1760 (1998).
Brugnoni, R., Galantini, S., Confalonieri, P., Balestrini, M. R., Cornelio, F. & Mantegazza, R. Identification of three novel mutations in the major human skeletal muscle chloride channel gene (CLCN1), causing myotonia congenita. Hum. Mutat. 14, 447 (1999).
de Diego, C., Gámez, J., Plassart-Schiess, E., Lasa, A., Del Río, E., Cervera, C. et al. Novel mutations in the muscle chloride channel CLCN1 gene causing myotonia congenita in Spanish families. J. Neurol. 246, 825–829 (1999).
Esteban, J., Neumeyer, A. M., McKenna-Yasek, D. & Brown, R. H. Identification of two mutations and a polymorphism in the chloride channel CLCN-1 in patients with Becker’s generalized myotonia. Neurogenetics 1, 185–188 (1998).
Fialho, D., Schorge, S., Pucovska, U., Davies, N. P., Labrum, R., Haworth, A. et al. Chloride channel myotonia: exon 8 hot-spot for dominant-negative interactions. Brain 130, 3265–3274 (2007).
Mazón, M. J., Barros, F., De la Peña, P., Quesada, J. F., Escudero, A., Cobo, A. M. et al. Screening for mutations in Spanish families with myotonia. Functional analysis of novel mutations in CLCN1 gene. Neuromuscul. Disord. 22, 231–243 (2012).
Modoni, A., D’Amico, A., Dallapiccola, B., Mereu, M. L., Merlini, L., Pagliarani, S. et al. Low-rate repetitive nerve stimulation protocol in an Italian cohort of patients affected by recessive myotonia congenita. J. Clin. Neurophysiol. 28, 39–44 (2011).
Pusch, M. Myotonia caused by mutations in the muscle chloride channel gene CLCN1. Hum. Mutat. 19, 423–434 (2002).
Sangiuolo, F., Botta, A., Mesoraca, A., Servidei, S., Merlini, L., Fratta, G. et al. Identification of five new mutations and three novel polymorphisms in the muscle chloride channel gene (CLCN1) in 20 Italian patients with dominant and recessive myotonia congenita. Hum. Mutat. 11, 331 (1998).
Trip, J., Drost, G., Verbove, D. J., van der Kooi, A. J., Kuks, J. B., Notermans, N. C. et al. In tandem analysis of CLCN1 and SCN4A greatly enhances mutation detection in families with non-dystrophic myotonia. Eur. J. Hum. Genet. 16, 921–929 (2008).
Wu, F. F., Ryan, A., Devaney, J., Warnstedt, M., Korade-Mirnics, Z., Poser, B. et al. Novel CLCN1 mutations with unique clinical and electrophysiological consequences. Brain 125, 2392–2407 (2002).
Leiden Open Variation Database. Chloride channel 1, skeletal muscle (CLCN1). http://chromium.liacs.nl/LOVD2/ (2012).
Plassart-Schiess, E., Gervais, A., Eymard, B., Lagueny, A., Pouget, J., Warter, J. M. et al. Novel muscle chloride channel (CLCN1) mutations in myotonia congenita with various modes of inheritance including incomplete dominance and penetrance. Neurology 50, 1176–1179 (1998).
Sloan Brown, K. & George, A. L. Inheritance of three distinct muscle chloride channel gene (CLCN1) mutations in a single recessive myotonia congenita family. Neurology 48, 542–543 (1997).
Sali, A., Potterton, L., Yuan, F., van Vlijmen, H. & Karplus, M. Evaluation of comparative protein modeling by MODELLER. Proteins 23, 318–326 (1995).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK—a program to check the stereochemical quality of protein structures. J. App. Cryst. 26, 283–291 (1993).
Zhang, C., Li, W. H., Krainer, A. R. & Zhang, M. Q. RNA landscape of evolution for optimal exon and intron discrimination. Proc. Natl. Acad. Sci. USA 105, 5797–5802 (2008).
Sironi, M., Menozzi, G., Riva, L., Cagliani, R., Comi, G. P., Bresolin, N. et al. Silencer elements as possible inhibitors of pseudoexon splicing. Nucleic Acid Res. 32, 1783–1791 (2004).
Shalata, A., Furman, H., Adir, V., Adir, N., Hujeirat, Y., Shalev, S. A. et al. Myotonia congenita in a large consanguineous Arab family: insight into the clinical spectrum of carriers and double heterozygotes of a novel mutation in the chloride channel CLCN1 gene. Muscle Nerve 41, 464–469 (2010).
Castro, P. M., Real, R., Leão, M. & Silveira, F. Co-dominant expression of a new mutation in myotonia congenita. Abstract. 21st Meeting of the European Neurological Society, (Lisbon, Portugal, 2011).
George, A. L. Jr, Sloan-Brown, K., Fenichel, G. M., Mitchell, G. A., Spiegel, R. & Pascuzzi, R. M. Nonsense and missense mutations of the muscle chloride channel gene in patients with myotonia congenita. Hum. Mol. Genet. 3, 2071–2072 (1994).
Lehmann-Horn, F., Mailander, V., Heine, R. & George, A. L. Myotonia levior is a chloride channel disorder. Hum. Mol. Genet. 4, 1397–1402 (1995).
Dupre, N., Chrestian, N., Bouchard, J. P., Rossignol, E., Brunet, D., Sternberg, D. et al. Clinical, electrophysiologic, and genetic study of non-dystrophic myotonia in French-Canadians. Neuromuscul. Disord. 19, 330–334 (2009).
Sun, C., Tranebjaerg, L., Torbergsen, T., Holmgren, G. & Van Ghelue, M. Spectrum of CLCN1 mutations in patients with myotonia congenita in Northern Scandinavia. Eur. J. Hum. Genet. 9, 903–909 (2001).
Fahlke, C. Molecular mechanisms of ion conduction in ClC-type chloride channels: lessons from disease-causing mutations. Kidney Int. 57, 780–786 (2000).
Meyer-Kleine, C., Steinmeyer, K., Ricker, K., Jentsch, T. J. & Koch, M. C. Spectrum of mutations in the major human skeletal muscle chloride channel gene (CLCN1) leading to myotonia. Am. J. Hum. Genet. 57, 1325–1334 (1995).
Zhang, J., Bendahhou, S., Sanguinetti, M. C. & Ptacek, L. J. Functional consequences of chloride channel gene (CLCN1) mutations causing myotonia congenita. Neurology 54, 937–942 (2000).
Fahlke, C., Desai, R. R., Gillani, N. & George, A. L. Jr. Residues lining the inner pore vestibule of human muscle chloride channels. J. Biol. Chem. 276, 1759–1765 (2001).
Desaphy, J.-F., Rolland, J.-F., Valente, E. M., LoMonaco, M. & Conte Camerino, D. Functional alteration of ClC-1 channel mutants associated with transient weakness in myotonia congenita. Biophys. J. 92, 273a (2007).
Hsiao, K. M., Huang, R. Y., Tang, P. H. & Lin, M. J. Functional study of CLC-1 mutants expressed in Xenopus oocytes reveals that a C-terminal region Thr891-Ser892-Thr893 is responsible for the effects of protein kinase C activator. Cell Physiol. Biochem. 25, 687–694 (2010).
Bennetts, B., Rychkov, G. Y., Ng, H. L., Morton, C. J., Stapleton, D., Parker, M. W. et al. Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J. Biol. Chem. 280, 32452–32458 (2005).
Macias, M. J., Teijido, O., Zifarelli, G., Martin, P., Ramirez-Espain, X., Zorzano, A. et al. Myotonia-related mutations in the distal C-terminus of ClC-1 and ClC-0 chloride channels affect the structure of a poly-proline helix. Biochem. J. 403, 79–87 (2007).
Ma, L., Rychkova, G. Y. & Bretag, A. H. Functional study of cytoplasmic loops of human skeletal muscle chloride channel, hClC-1. Int. J. Biochem. Cell Biol. 41, 1402–1409 (2009).
Tan, S. V., Matthews, E., Barber, M., Burge, J. A., Rajakulendran, S., Fialho, D. et al. Refined exercise testing can aid DNA-based diagnosis in muscle channelopathies. Ann. Neurol. 69, 328–340 (2011).
Raja Rayan, D. L., Haworth, A., Sud, R., Matthews, E., Fialho, D., Burge, J. et al. A new explanation for recessive myotonia congenita: Exon deletions and duplications in CLCN1. Neurology 78, 1953–1958 (2012).
Acknowledgements
We are grateful to the MC patients and their families for their cooperation, and to the doctors who provided the blood samples for the study. This work was supported by the Italian Ministry of Health (Grant No. 1580433).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Brugnoni, R., Kapetis, D., Imbrici, P. et al. A large cohort of myotonia congenita probands: novel mutations and a high-frequency mutation region in exons 4 and 5 of the CLCN1 gene. J Hum Genet 58, 581–587 (2013). https://doi.org/10.1038/jhg.2013.58
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2013.58
Keywords
This article is cited by
-
Community data-driven approach to identify pathogenic founder variants for pan-ethnic carrier screening panels
Human Genomics (2023)
-
Non-dystrophic myotonias: clinical and mutation spectrum of 70 German patients
Journal of Neurology (2021)
-
Association of Three Different Mutations in the CLCN1 Gene Modulating the Phenotype in a Consanguineous Family with Myotonia Congenita
Journal of Molecular Neuroscience (2021)
-
Skeletal muscle ClC-1 chloride channels in health and diseases
Pflügers Archiv - European Journal of Physiology (2020)
-
Becker’s myotonia: novel mutations and clinical variability in patients born to consanguineous parents
Acta Neurologica Belgica (2018)